Parkinson’s disease is a chronic degenerative neurological disorder affecting millions globally. Its manifestation — the death of dopaminergic cells in the substantia nigra — leads to motor dysfunction, encompassing symptoms like shaking, rigidity, and gait abnormalities, as well as potential non-motor symptoms, including cognitive dysfunction. At PsychoGenics, our goal is to enable you to identify groundbreaking treatments for this debilitating disease.
This model, developed in collaboration with the Michael J. Fox Foundation, enables us to induce propagation of α-synuclein by striatal injection of mouse preformed α-synuclein fibrils. α-synuclein is a key pathological feature of PD and contributes to disease progression.
Synthetic alpha-synuclein pre-formed fibrils (PFF) are capable of ‘seeding’ and propagating alpha synuclein pathology in mice. Intrastriatal PFF inoculation leads to a significant number of alpha-synuclein aggregates that spread over time from the striatum to other brain regions, including dopaminergic neurons of the substantia nigra. PFF inoculation leads to the appearance of Lewy body and Lewy neurite-like inclusions. Over time, the number of fine structured neuritic inclusions reduced with associated neurodegeneration.
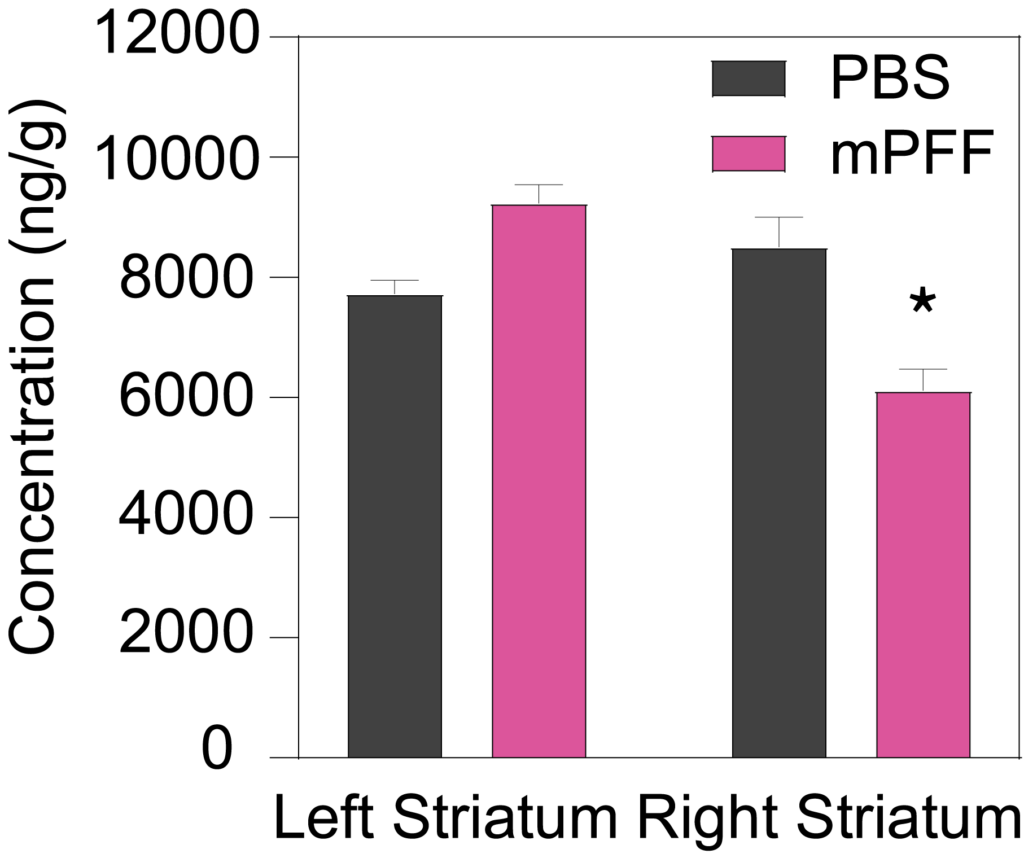
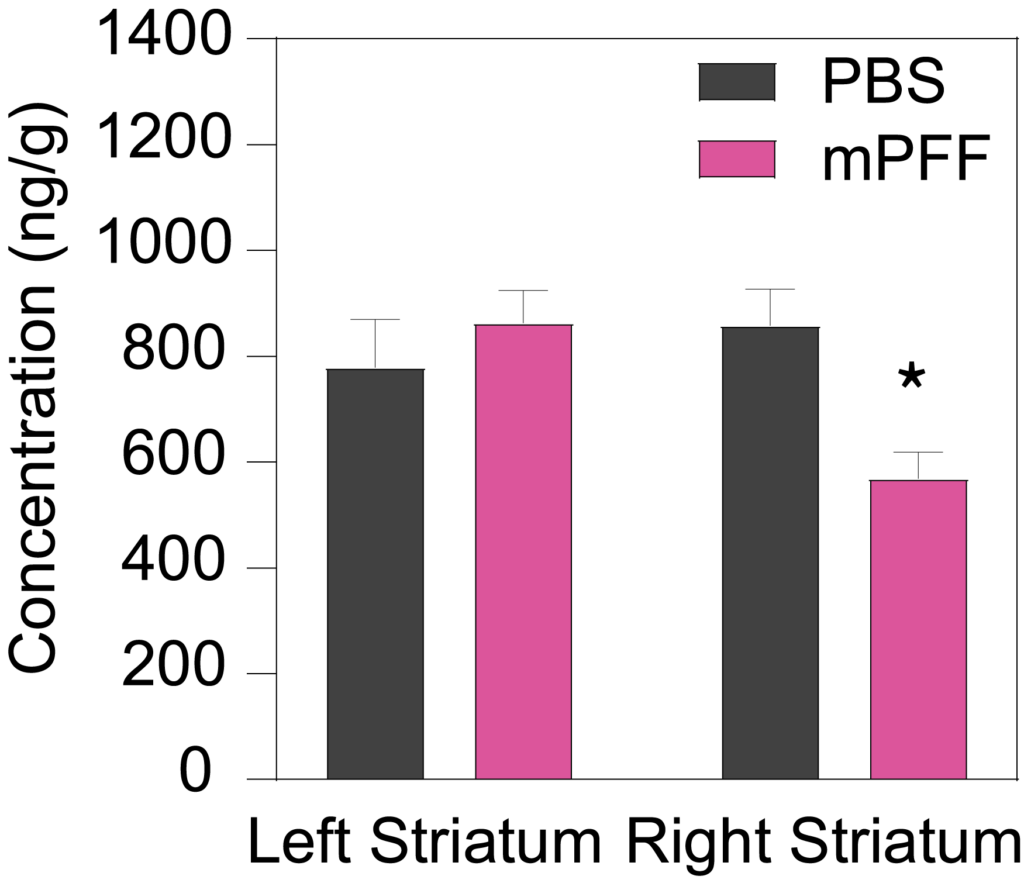
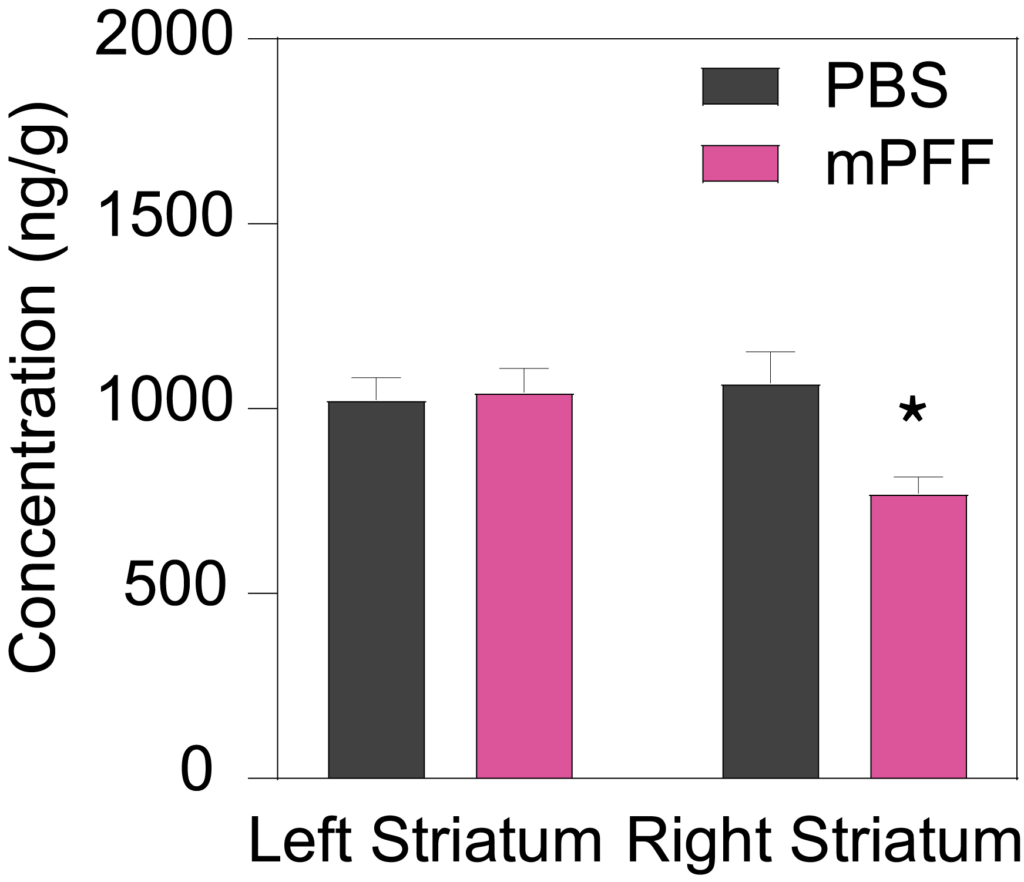
Male C57Bl/6J mice inoculated with PFF (5.0 µg) into the right striatum at 8 weeks of age show a significant decrease in ipsilateral, but not contralateral, striatal dopamine (Left), and the dopamine metabolites DOPAC (Middle) and HVA (Right) 90 days post-inoculation.
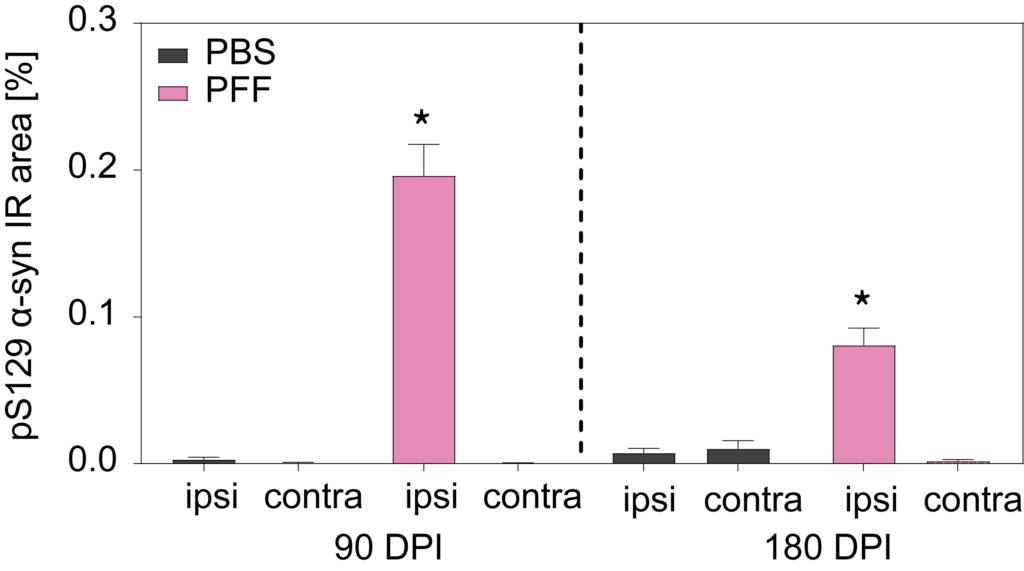
PFF-injected mice show increased pSer129 ɑ-synuclein aggregates in ipsilateral substantia nigra, but not contralateral. Total load of pSer129 aggregates can decrease between 90 and 180 DPI due to neuronal loss and subsequent cleavage.
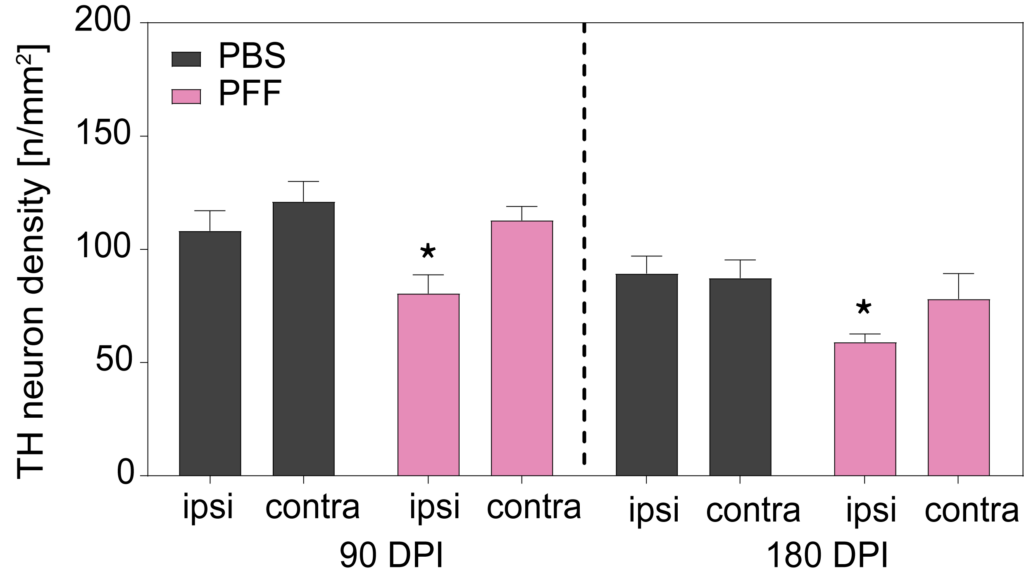
At 90- and 180-days post-PFF injection, a decrease in TH immunoreactivity and cell density was seen in ipsilateral substantia nigra but not contralateral, of PFF-injected mice.
The Thy-1-α-synuclein mouse model of PD overexpresses wild-type human α-synuclein under the Thy1 promoter in neurons. Alpha-synuclein is a presynaptic neuronal protein associated with PD progression. The model exhibits dopaminergic neuronal loss, α-synuclein aggregates, neuroinflammation, mitochondrial abnormalities, and motor impairment.
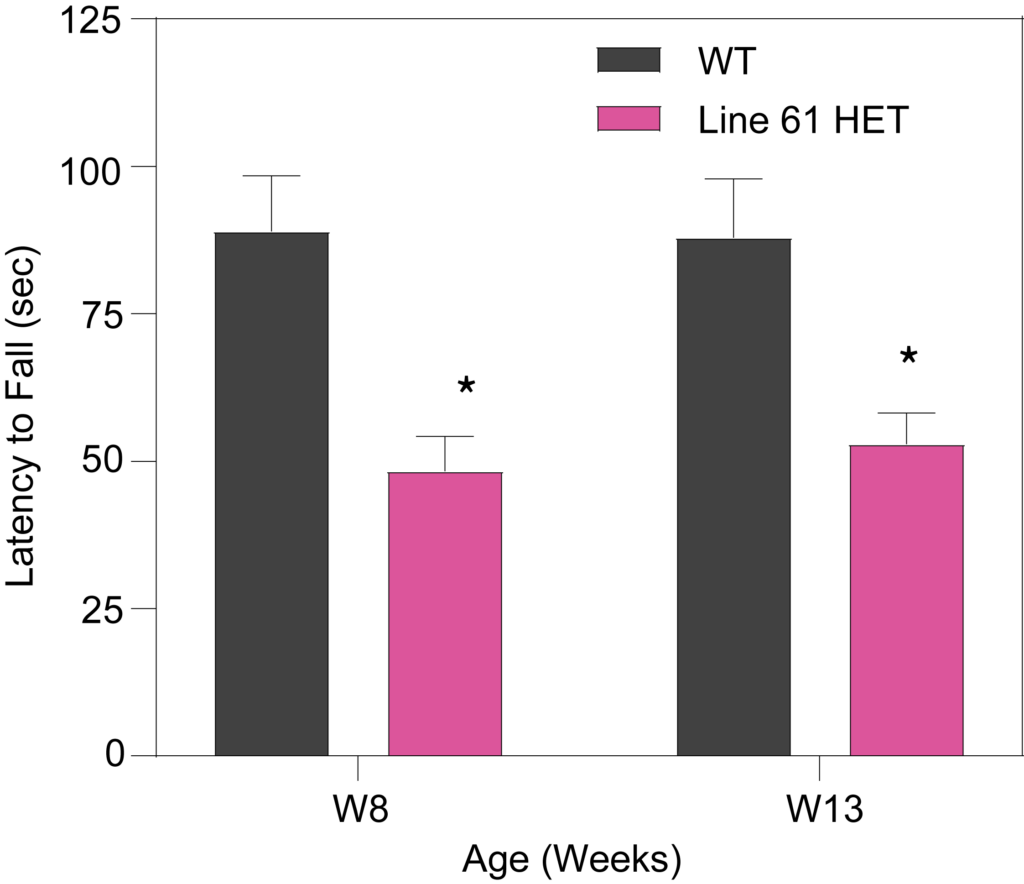
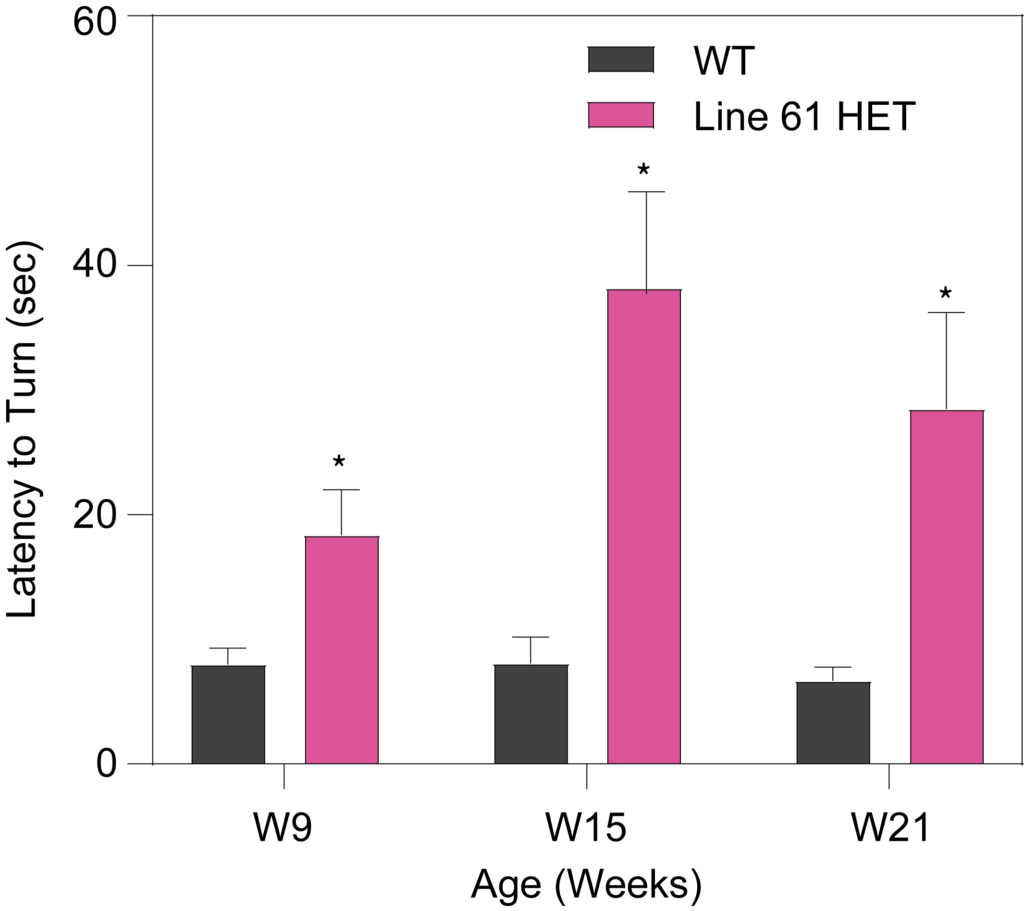
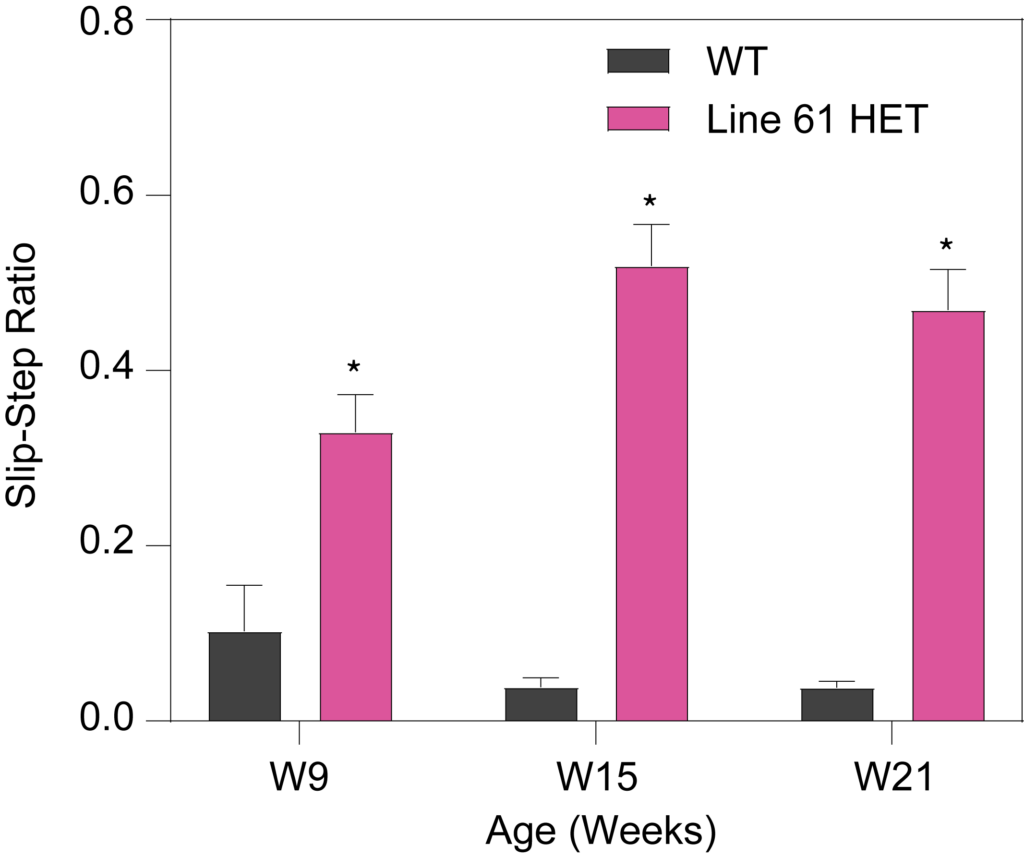
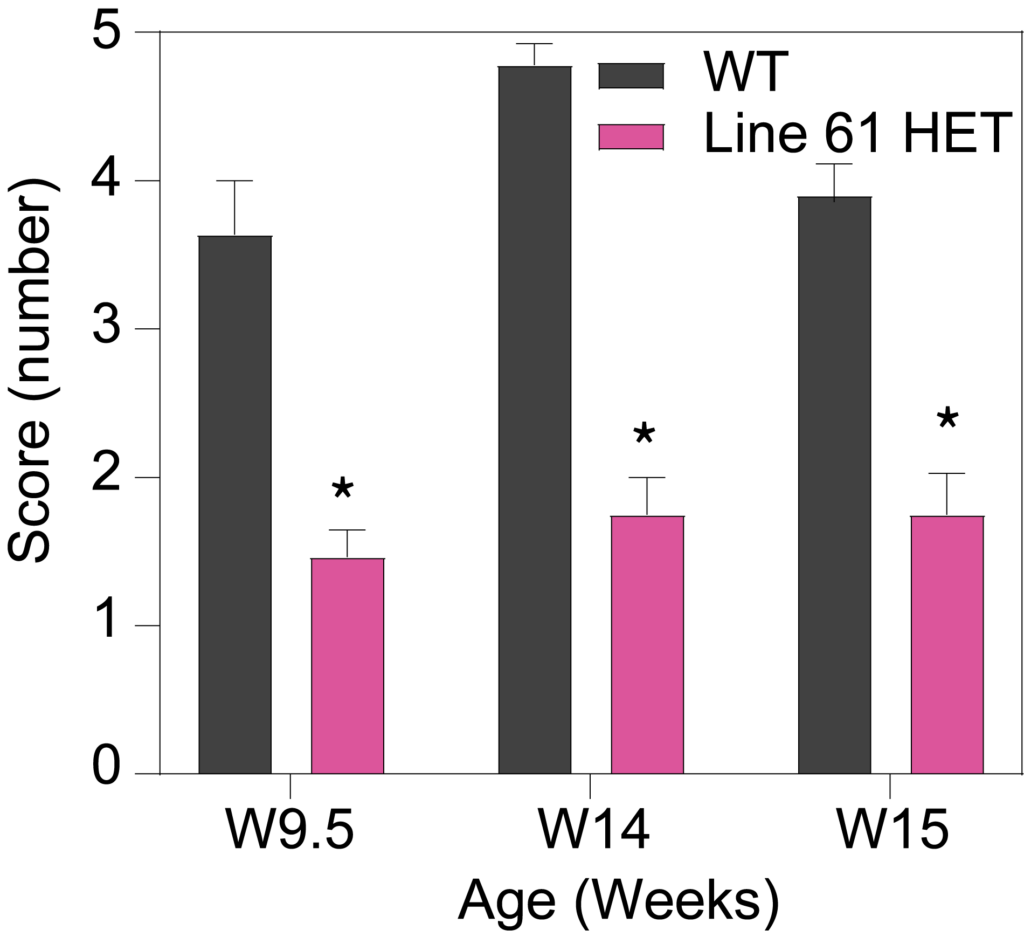
HET mice show deficits in rotarod performance (A), as well as longer latency to turn on the tapered balance beam (B) and show higher number of foot slips (C). Line 61 HET had significantly lower nest building scores compared to WT mice (D), suggesting a lack of dexterity and fine motor skills. The quality of the nest building was scored on a scale of 1 to 5, with a score of 5 representing a near-perfect nest using more than 90% of the nestlet.
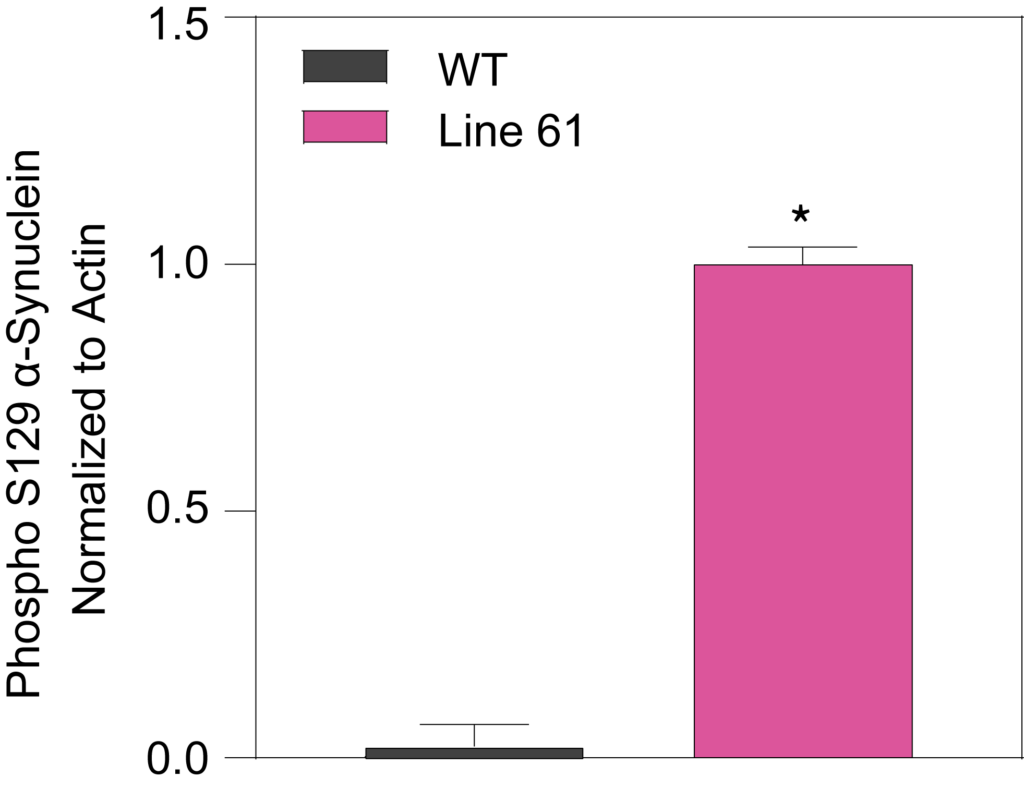
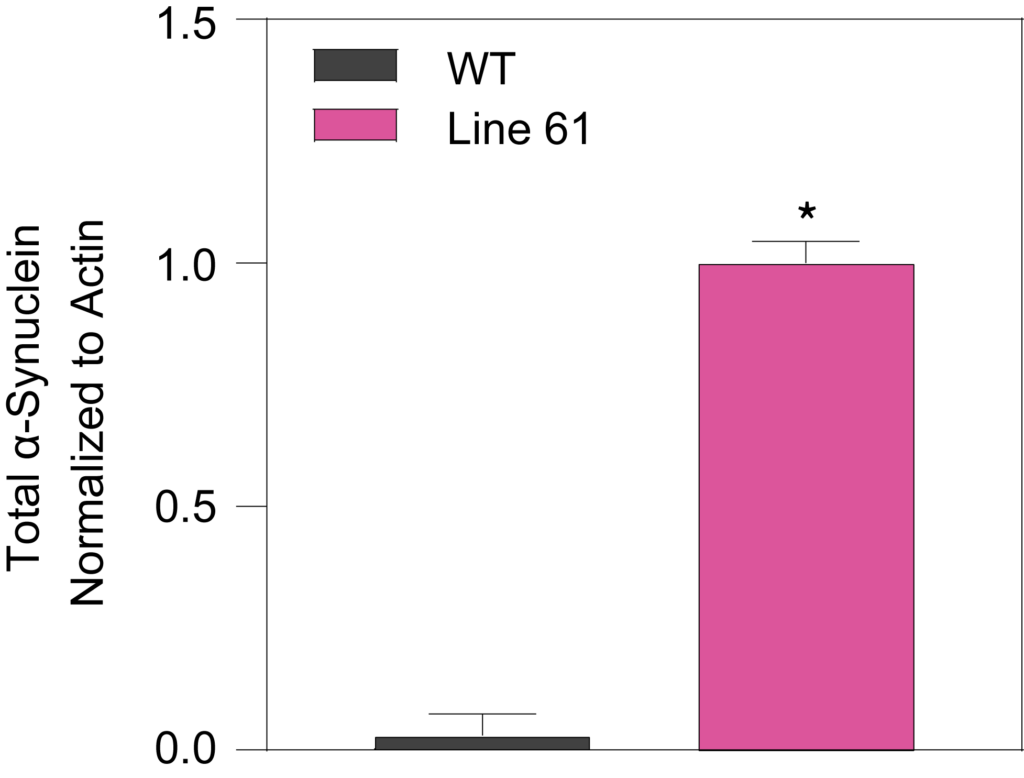
Phosphorylated Ser129 (Left) and total alpha synuclein proteins (Right) from midbrain samples of 15-week-old HET mice were significantly increased compared to WT mice.
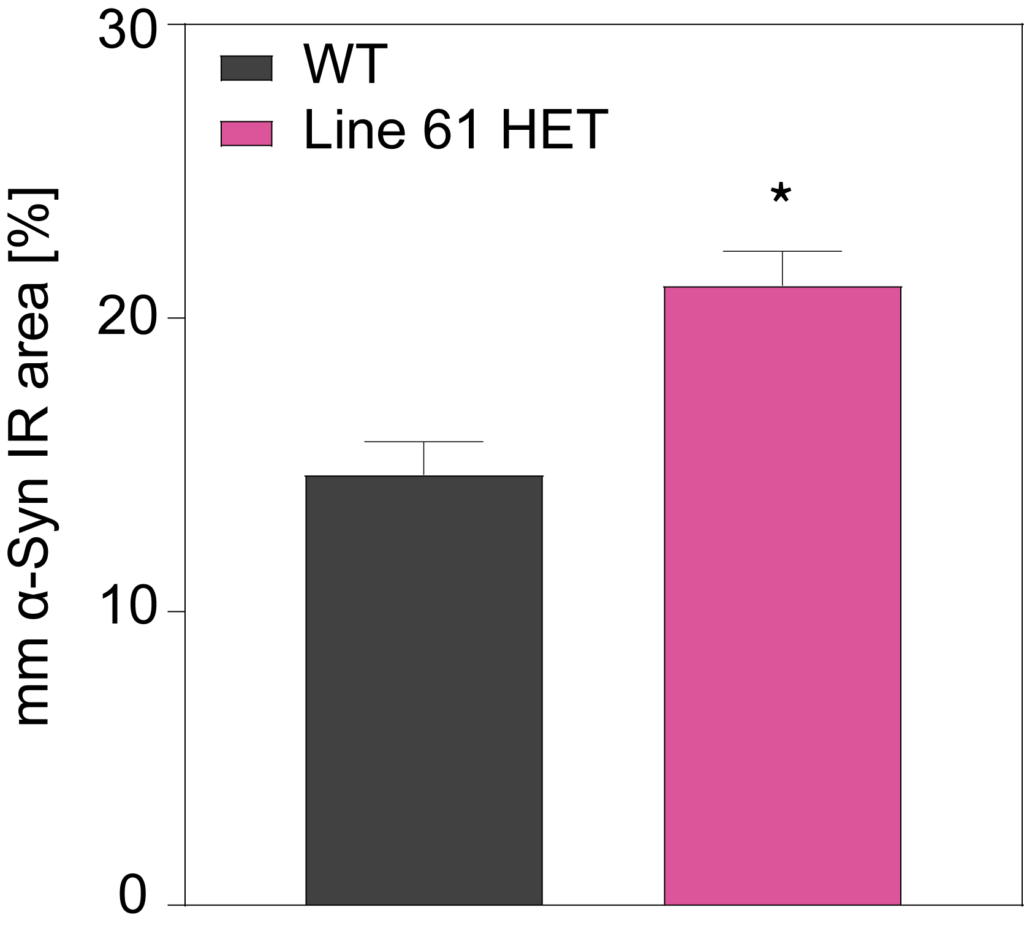
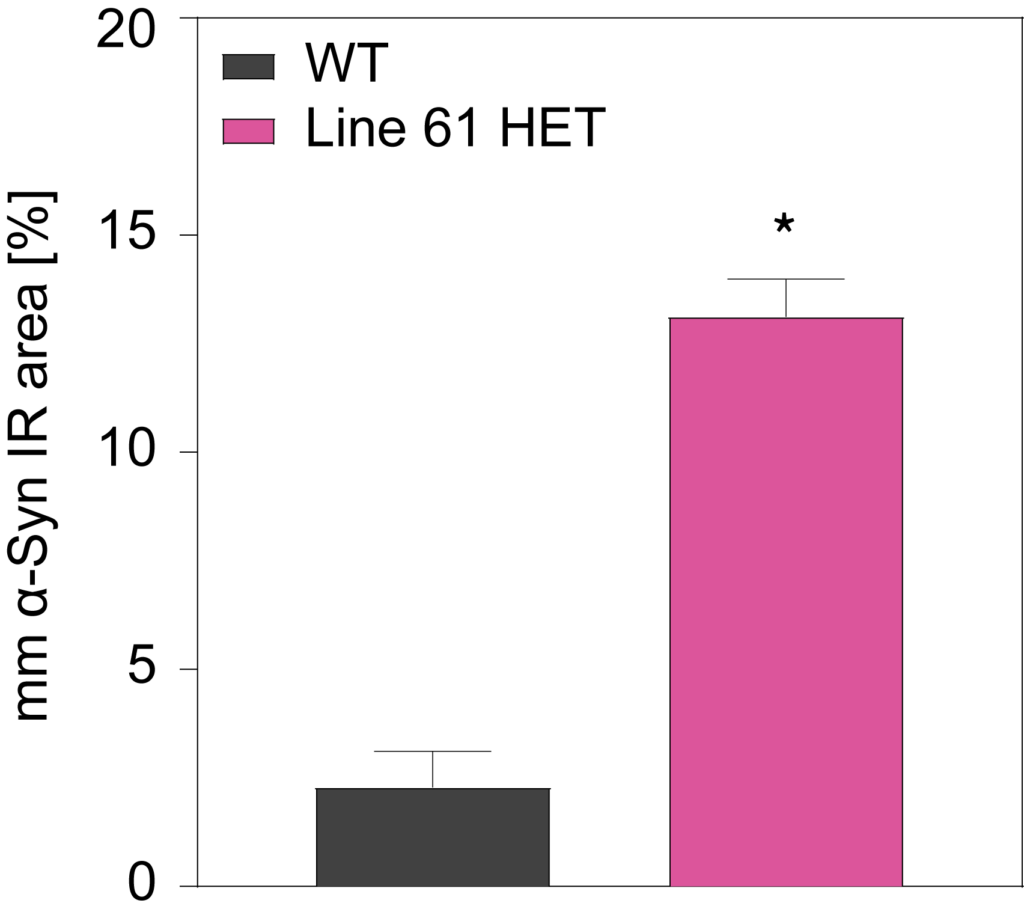
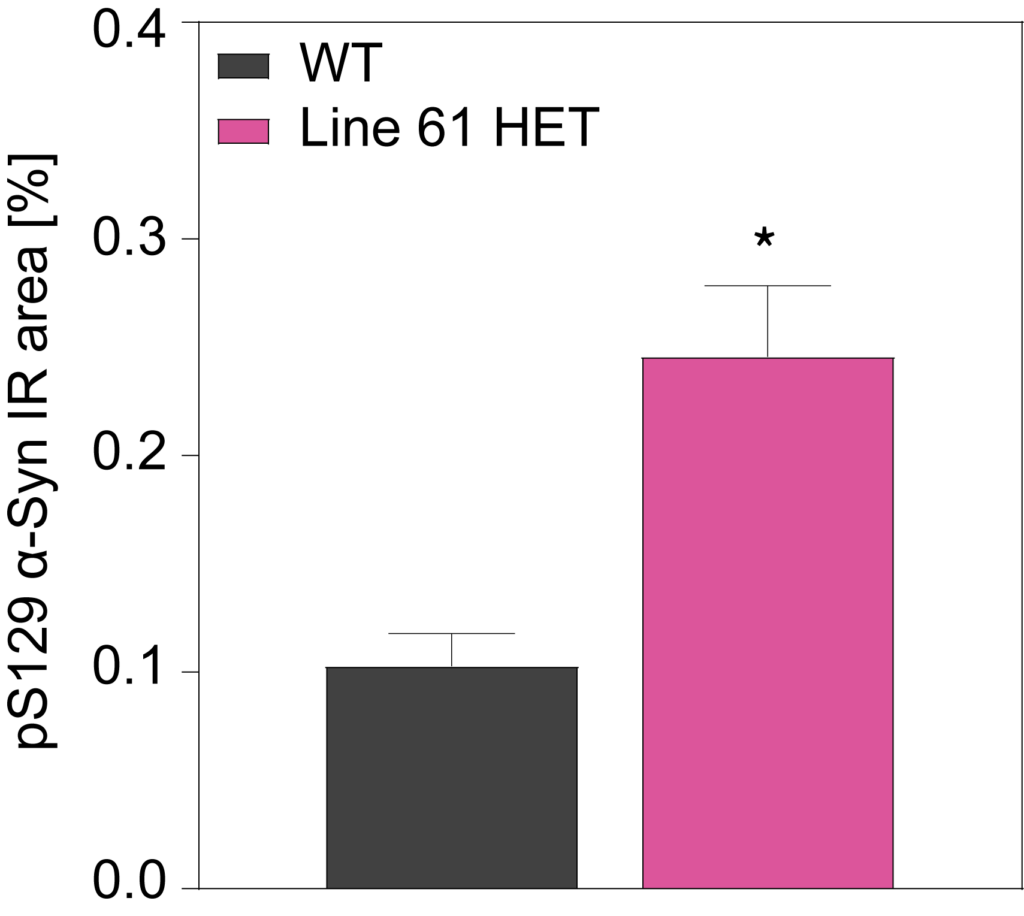
HET mice show a significant increase in multimeric alpha-synuclein compared to WT mice in cortex (A) and substantia nigra (B). They also show overexpression of hyperphosphorylated alpha-synuclein at residue Serine 129 (C).
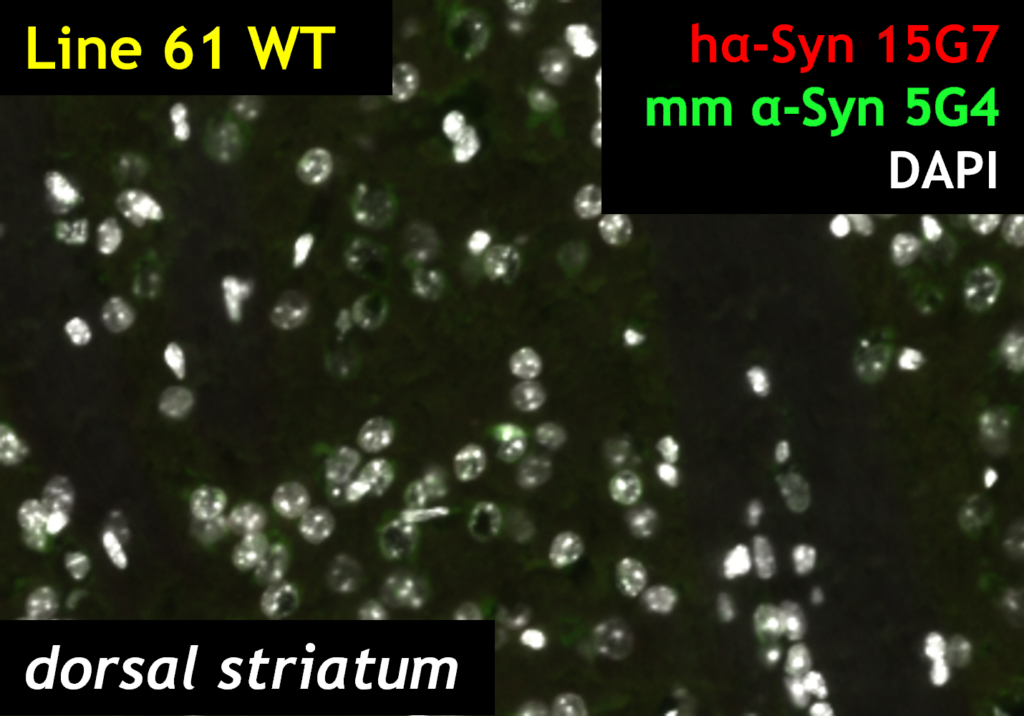
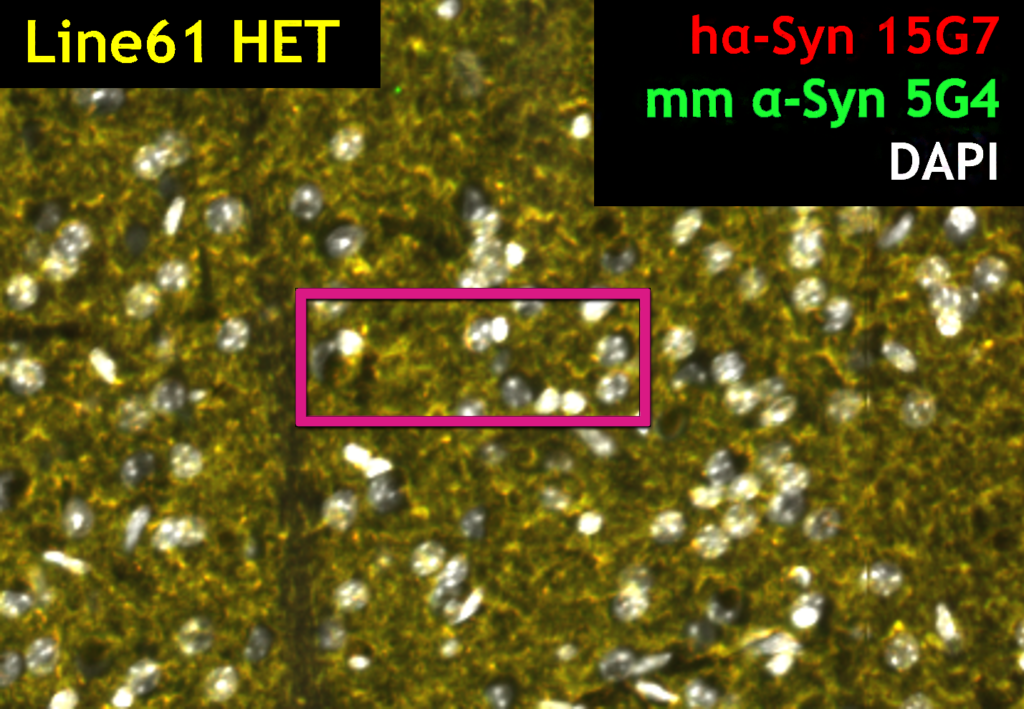

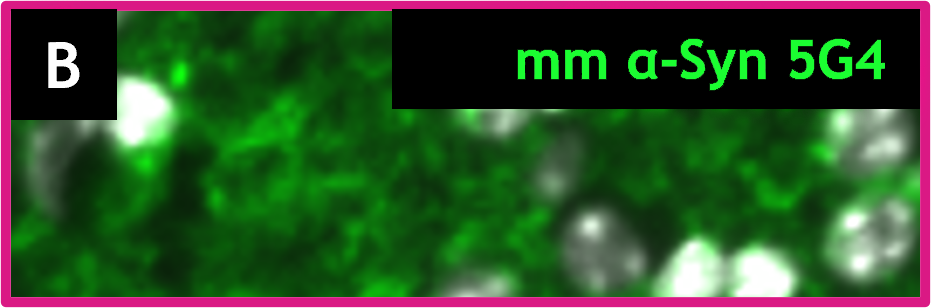

HET mice show over-expression of multimeric alpha-synuclein (green) as detected by 5G4, and human alpha-synuclein (red) as detected by 15G7 labeling. Insets show the positive labeling for each marker (A, 15G7; B, 5G4 and C, 15G7 + 5G4)
This model involves the injection of 6-OHDA into the rat striatum and medial forebrain bundle, resulting in the dramatic and permanent loss of dopaminergic neurons in the substantia nigra and subsequent motor deficits. This model has been used to assess neuroprotective agents and symptomatic treatments.
The 6-hydroxy-dopamine (6-OHDA) model simulates PD by targeting and destroying dopaminergic neurons in the brain. Injected 6-OHDA is selectively absorbed by dopamine transporters on neuronal membranes, leading to mitochondrial dysfunction and death. 6-OHDA is delivered unilaterally to the medial forebrain bundle, eliminating dopaminergic connections from the substantia nigra to the striatum. Characteristic outcomes of the model are contralateral motor impairment, decreased striatal dopamine levels, and reduced expression of tyrosine hydroxylase, a critical enzyme in dopamine synthesis.
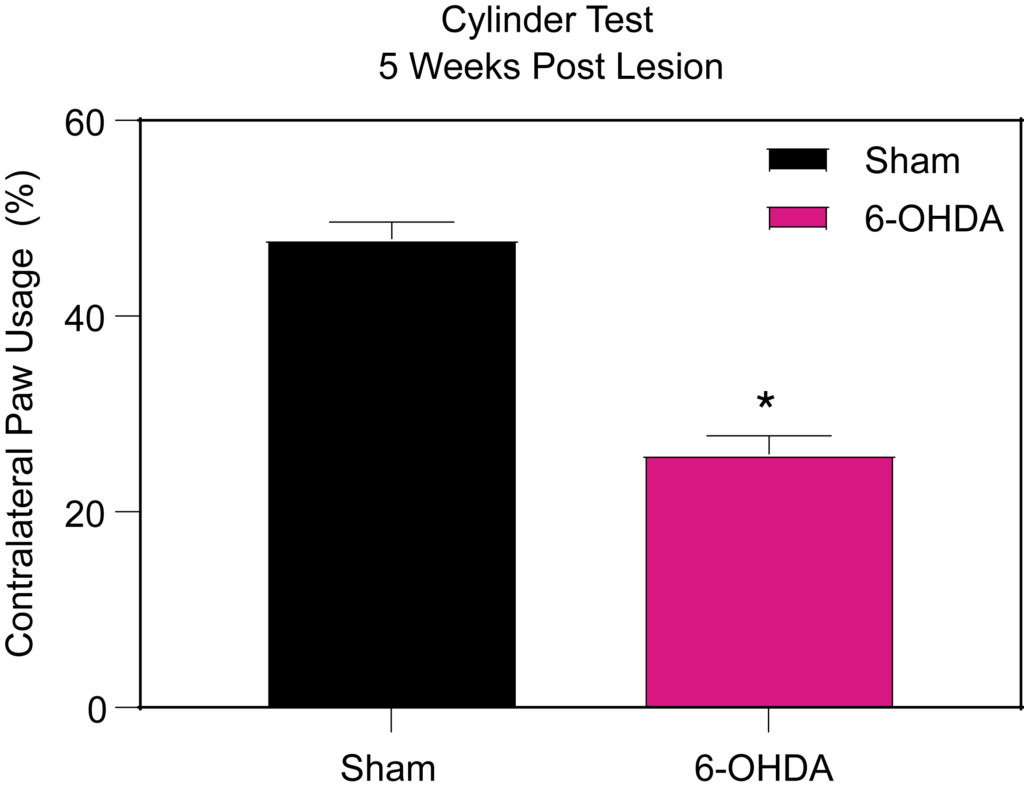
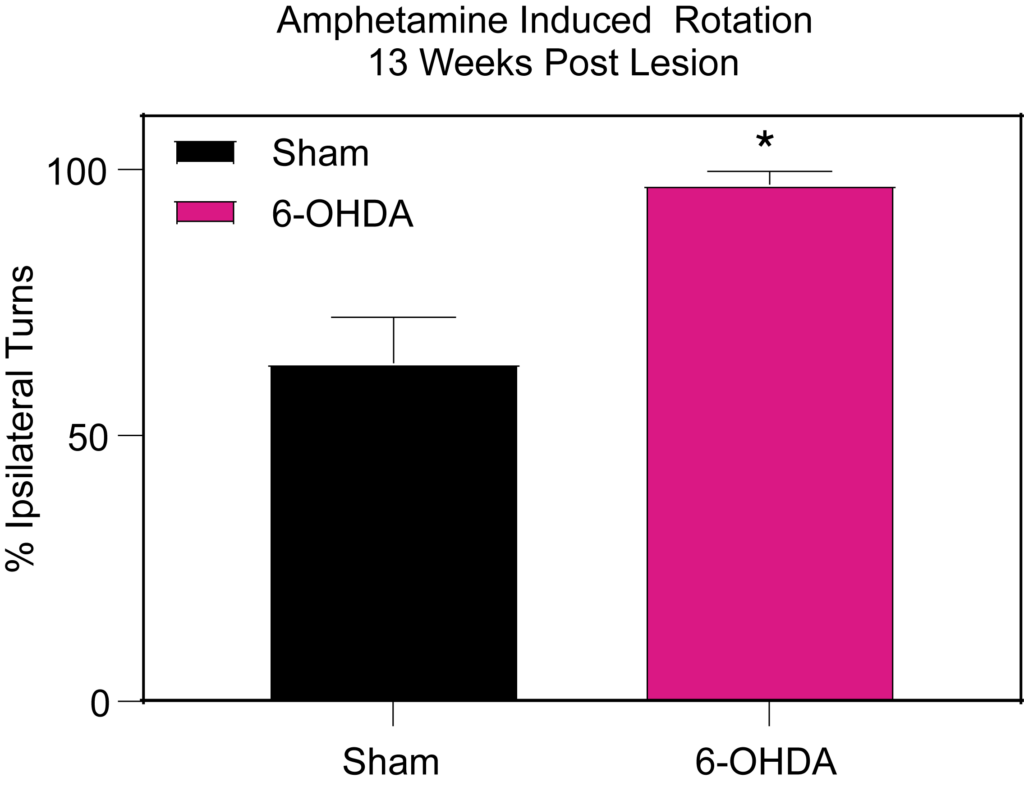
6-OHDA lesioned rats show impairment in (Left) contralateral paw use in the cylinder test 5 weeks post-lesion and (Right) increased ipsilateral rotations following amphetamine injection.
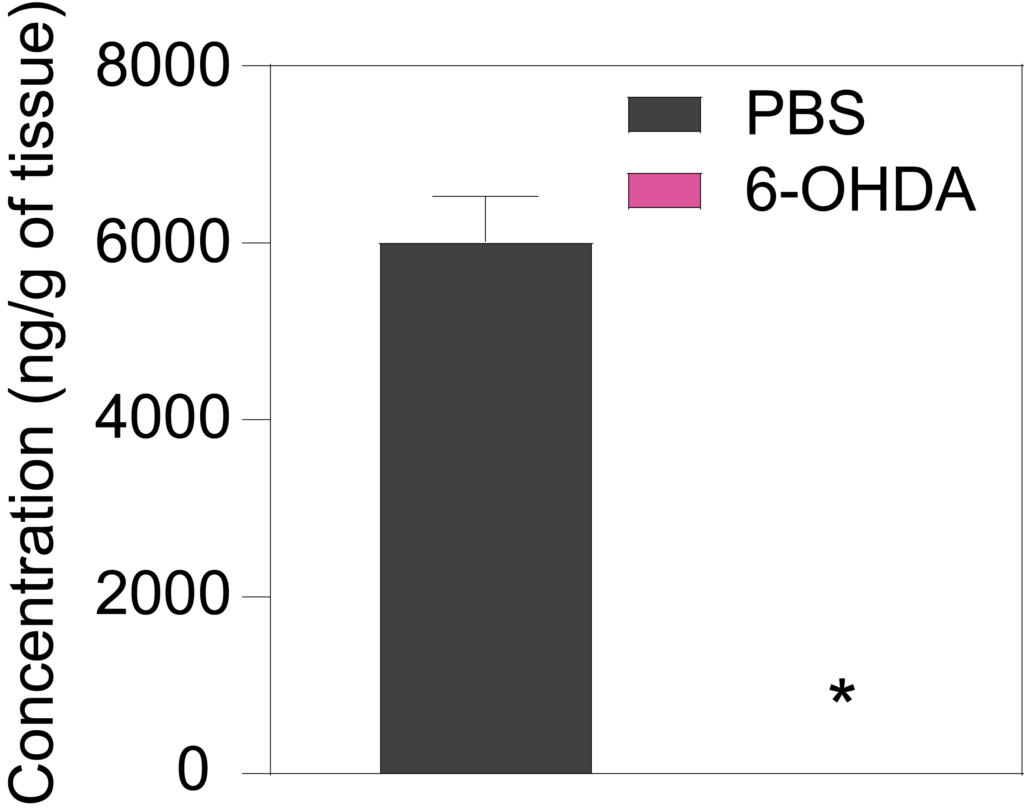
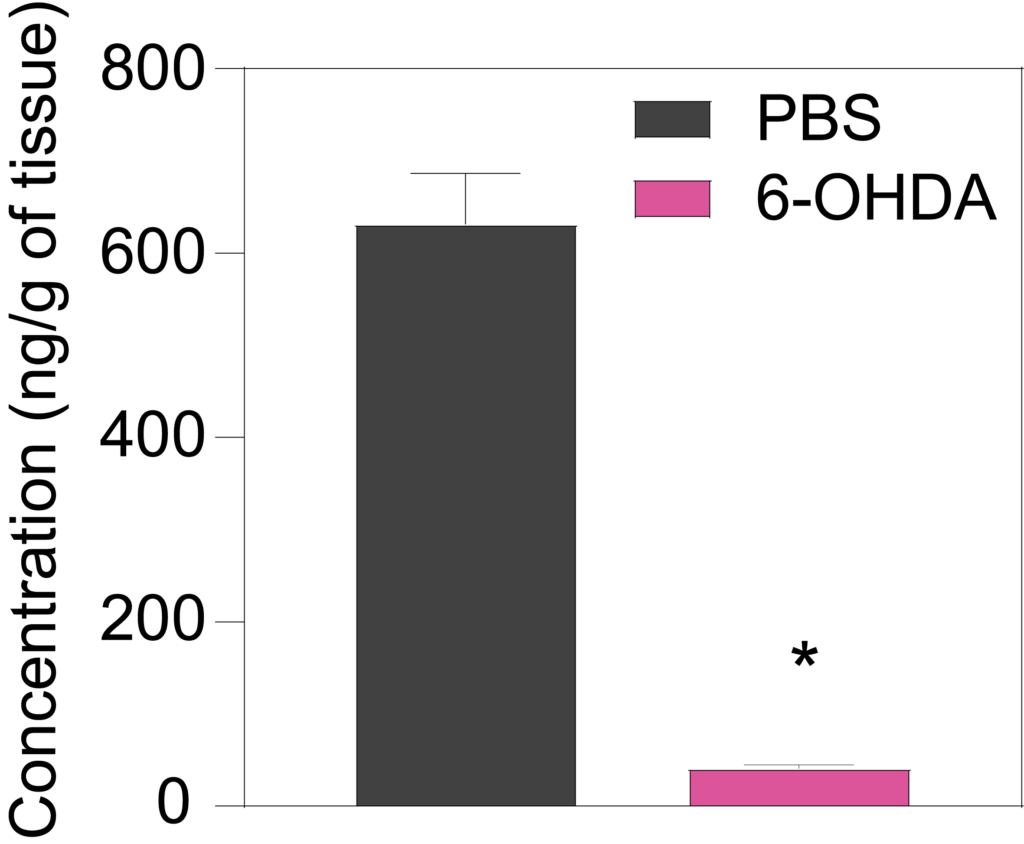
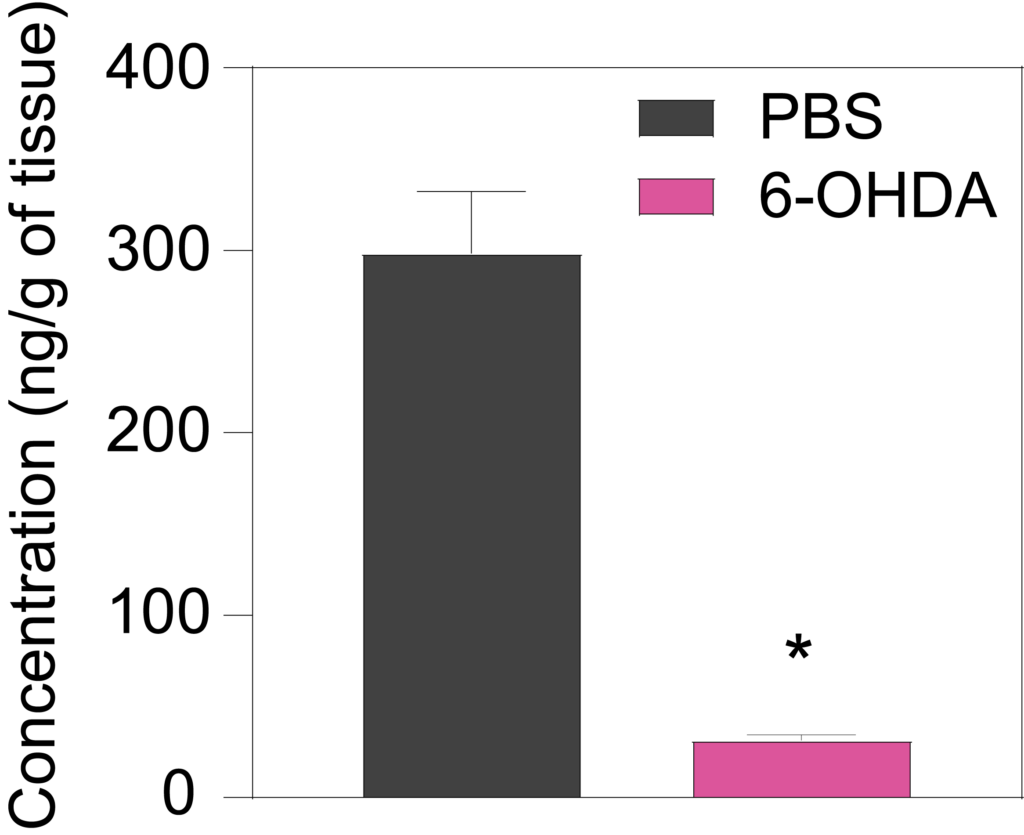
7 weeks a er lesion, 6-OHDA-treated rats show decreased striatal levels of dopamine (Left), DOPAC (Middle), and HVA (Right) in the ipsilateral striatum as compared to PBS-injected rats.
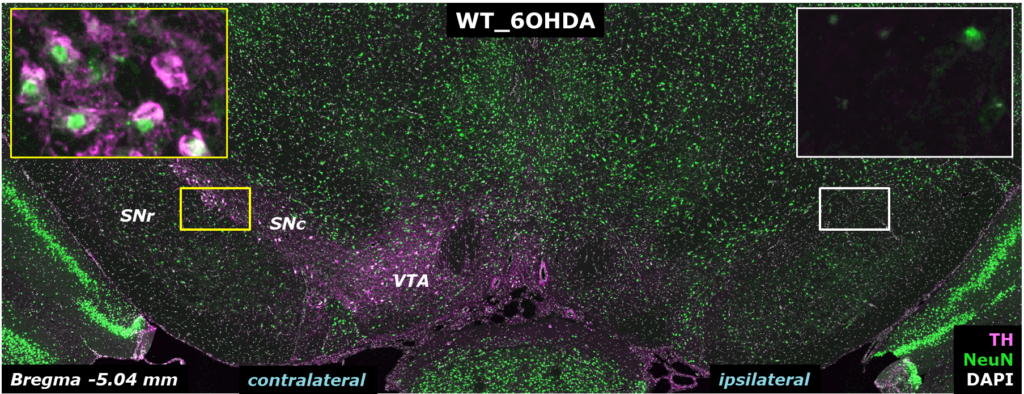
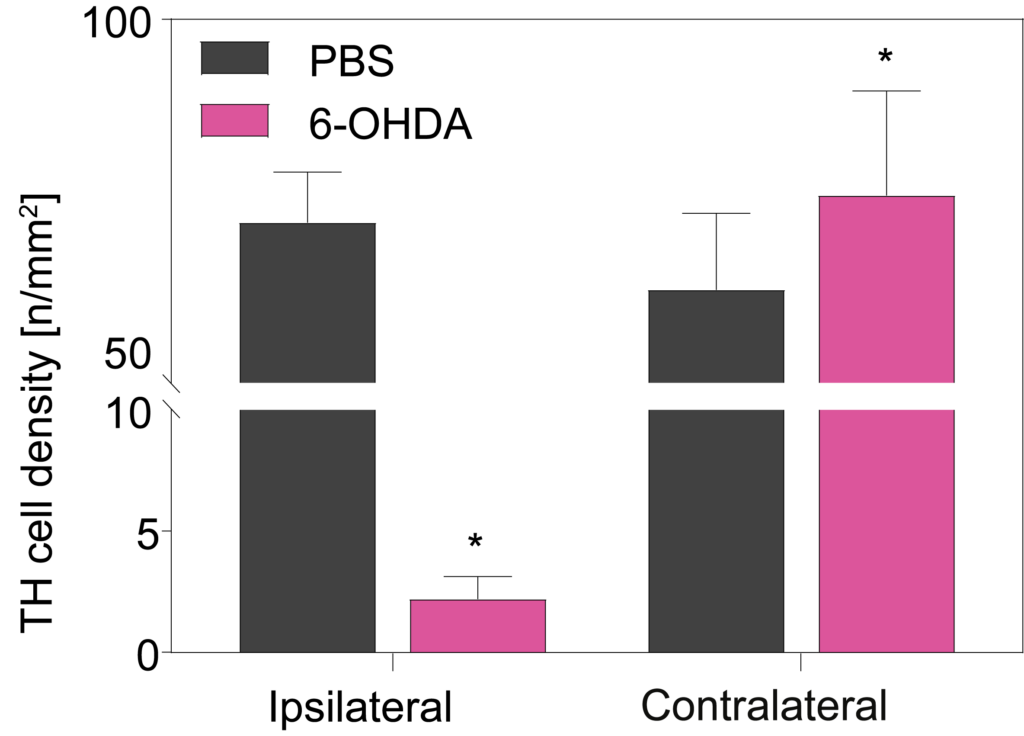
(Left) Tyrosine hydroxylase (TH, green) and neuronal nuclei labeling (NeuN, pink) counterstained with DAPI (white) in the substantia nigra of 6-OHDA-injected rats: 6-OHDA lesion leads to dramatic loss of TH positive projections in the hemisphere ipsilateral to the lesion. (Right) Measurement of TH dopaminergic cell density in the substantia nigra confirms the loss of dopaminergic neurons in the ipsilateral side of 6-OHDA-lesioned samples compared to contralateral or PBS controls.
MPTP, or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, is a neurotoxin that selectively damages dopaminergic neurons in the brain. MPTP is administered intraperitoneally and crosses the blood-brain barrier where it is taken up by dopamine transporters (DAT) on the membranes of dopaminergic neurons.
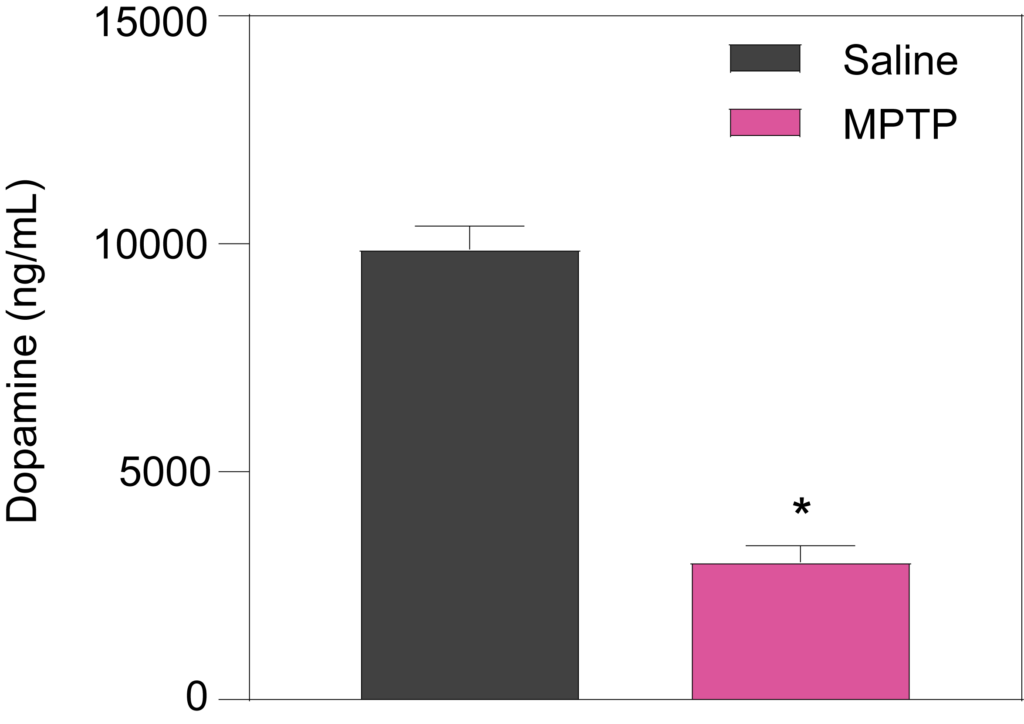
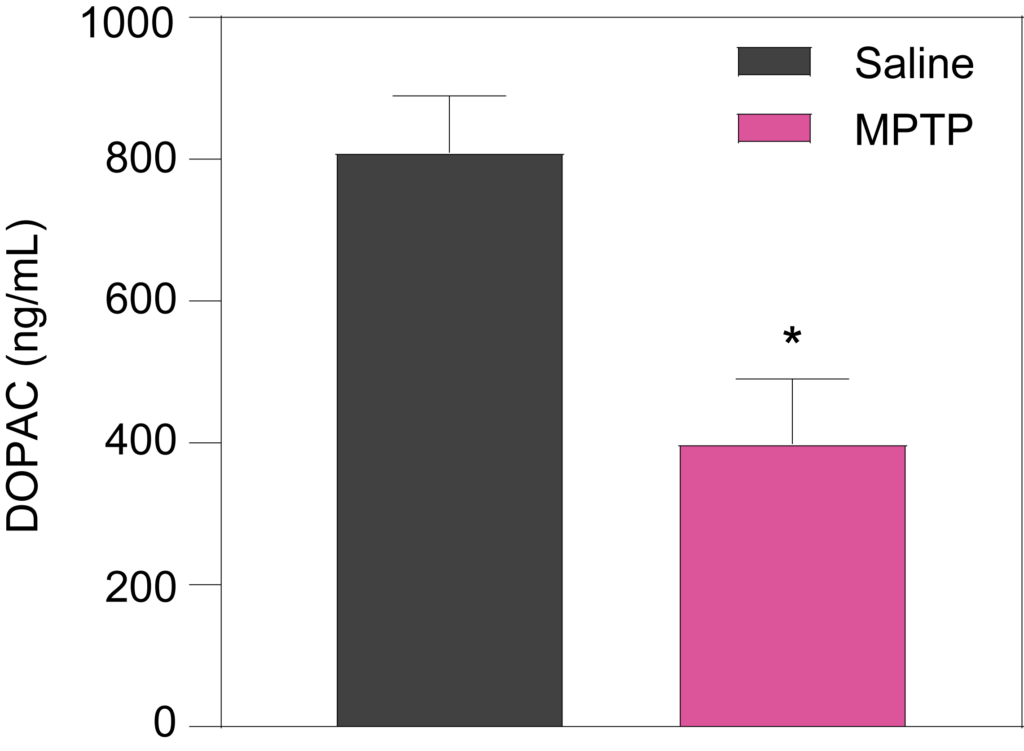
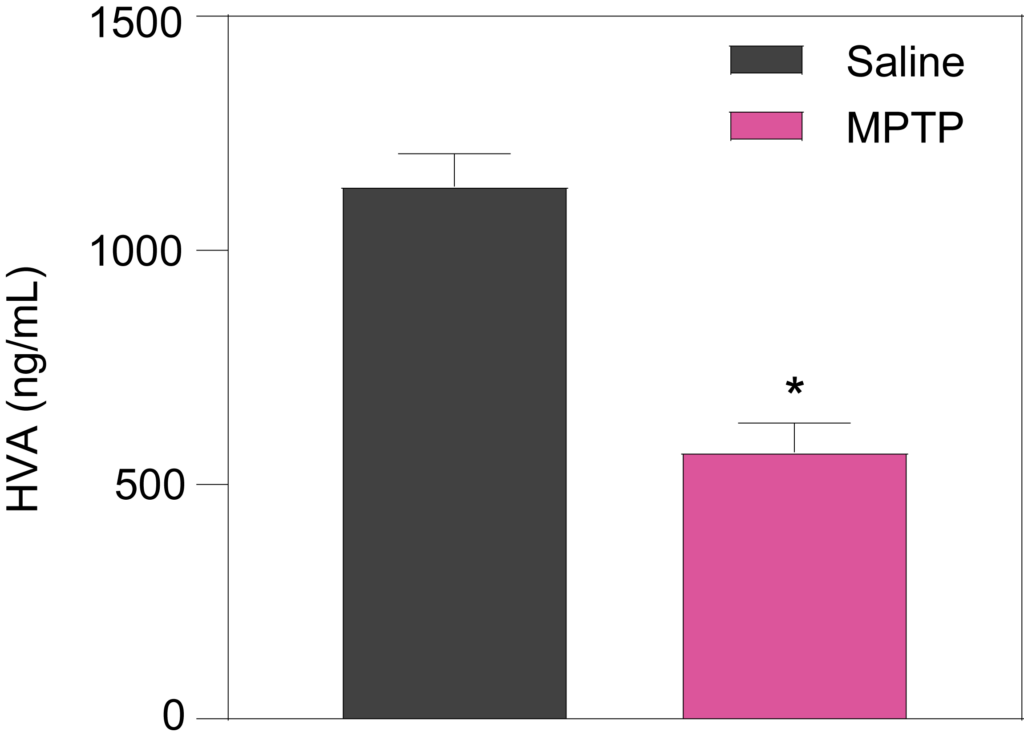
Striatal levels of dopamine (Left), and its metabolites DOPAC (Middle) and HVA (Right), are significantly decreased in mice treated with MPTP (25 mg/kg for 6 days).

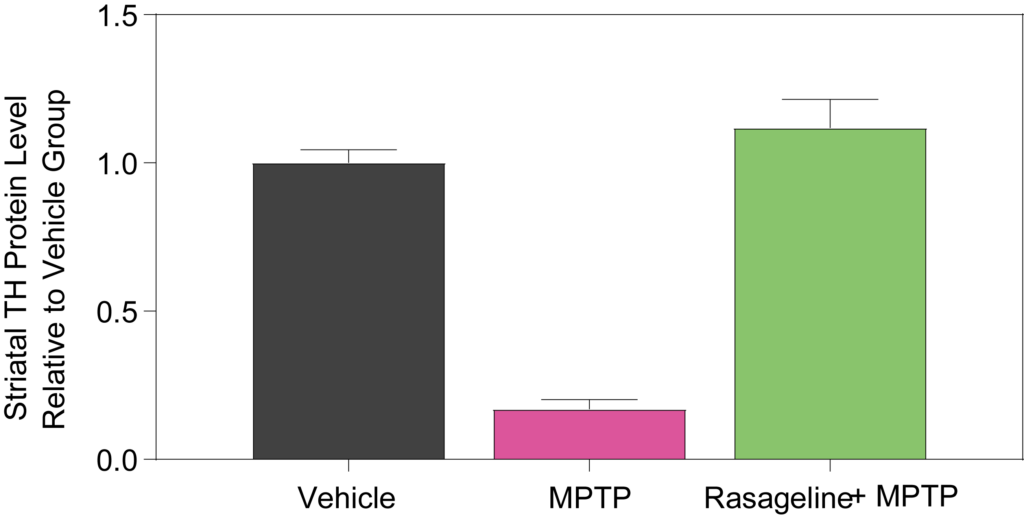
(Left) Western blot images of tyrosine hydroxylase (TH), an enzyme involved in the synthesis of dopamine, and GAPDH, a control housekeeper protein, scanned using the Typhoon scanner (Cytiva), shows a reduction in TH levels following treatment with MPTP and rescued with rasageline. (Right) Quantification of striatal TH levels are significantly decreased with MPTP treatment. Rasageline blocks MPTP toxicity and prevents the decrease in TH levels.
Explore our areas of neurodegenerative disease specialization: