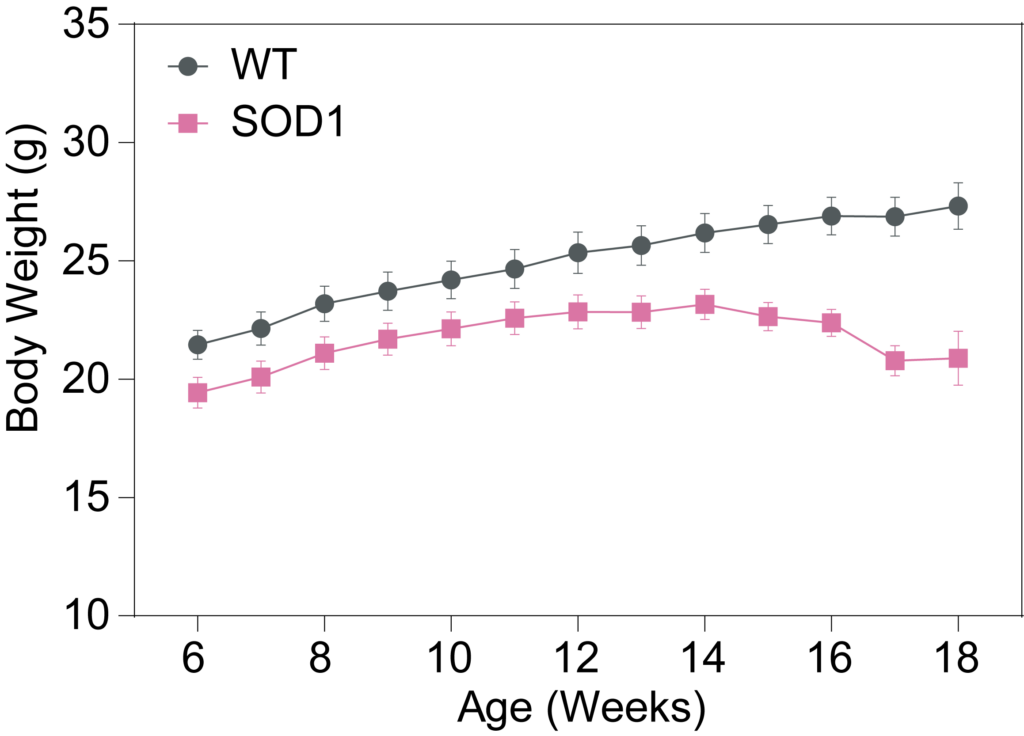
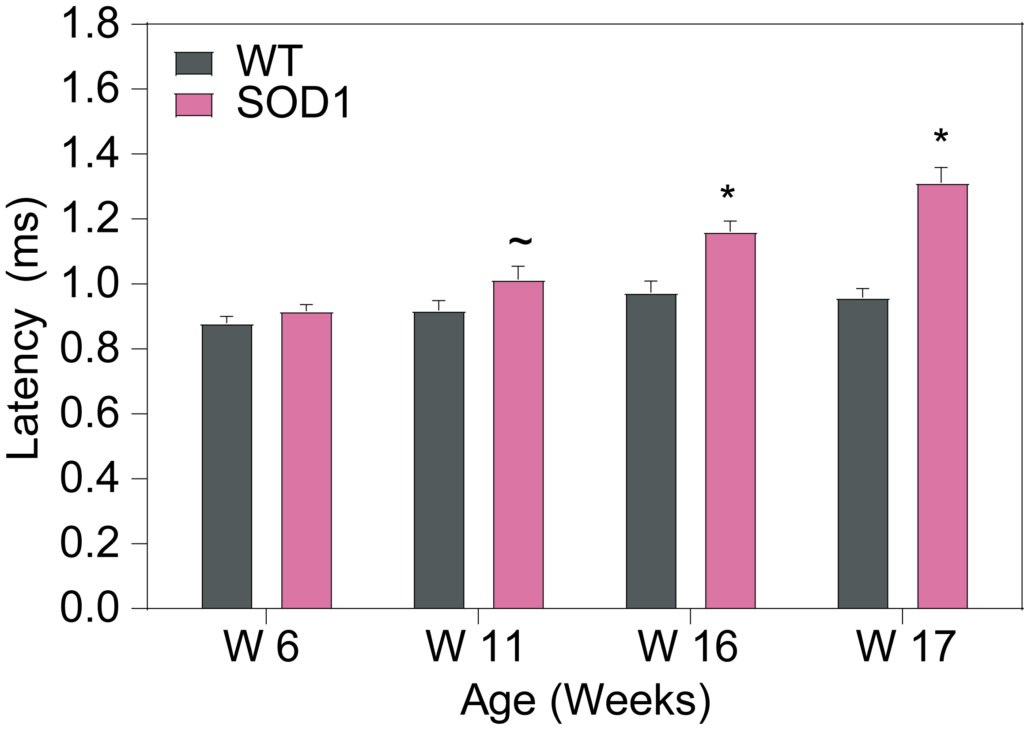
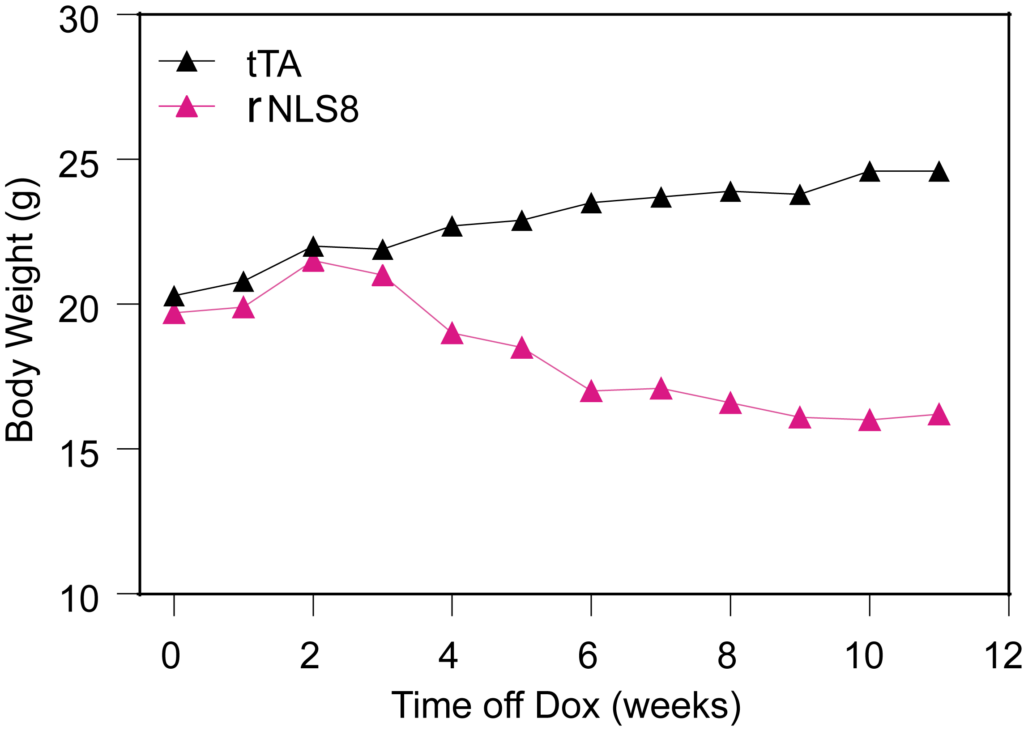
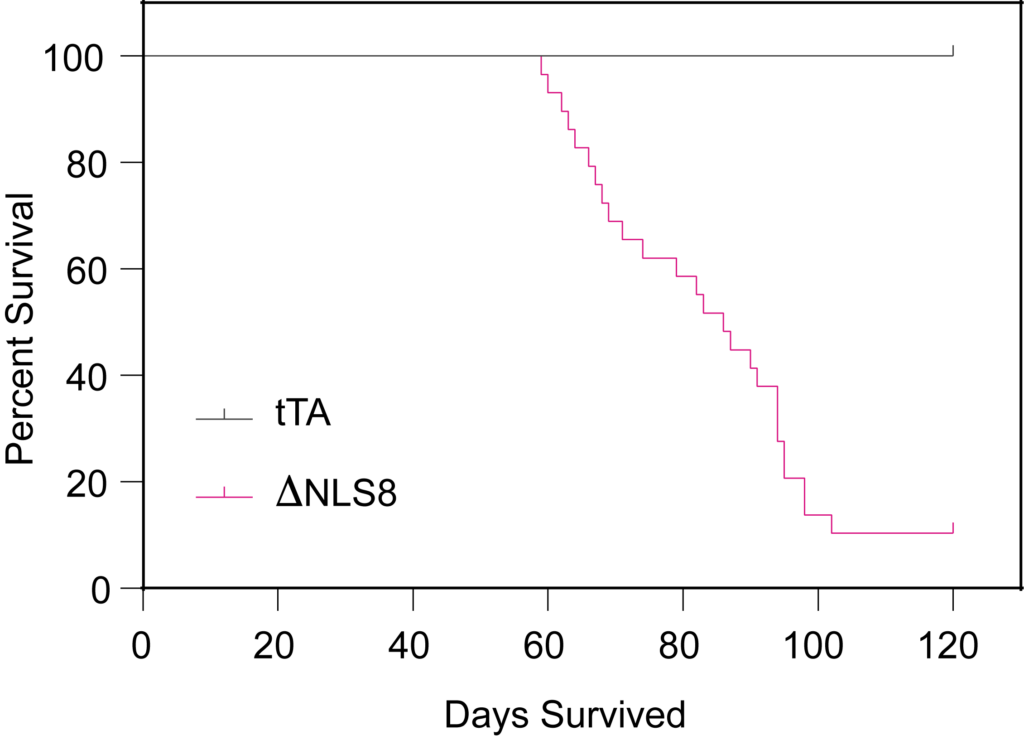
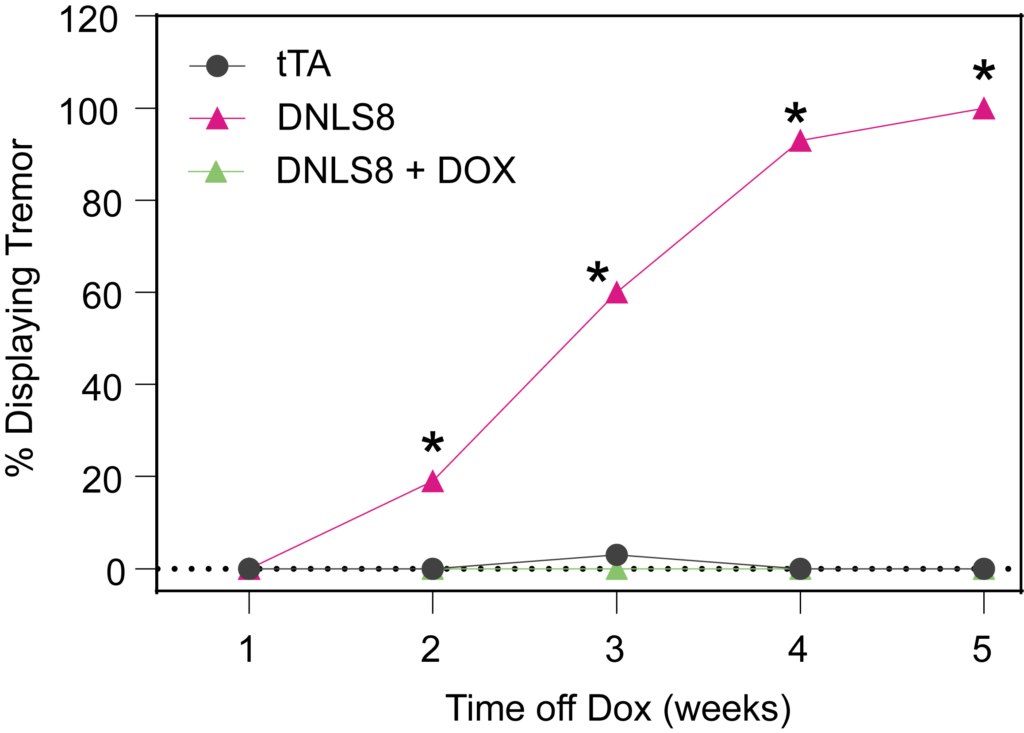
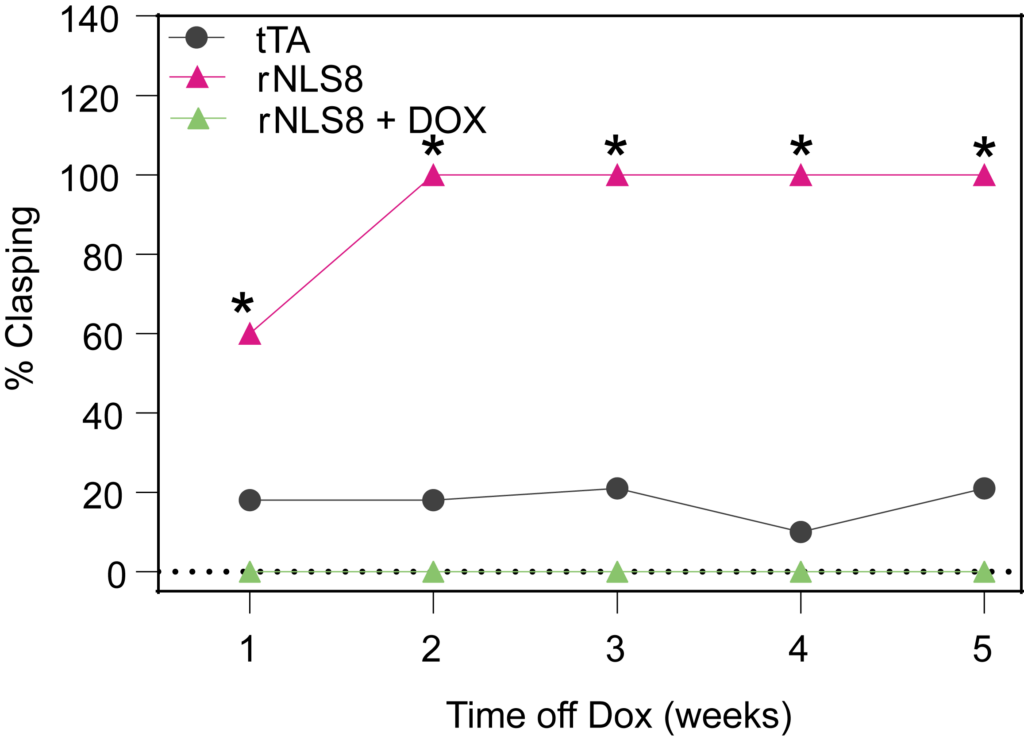
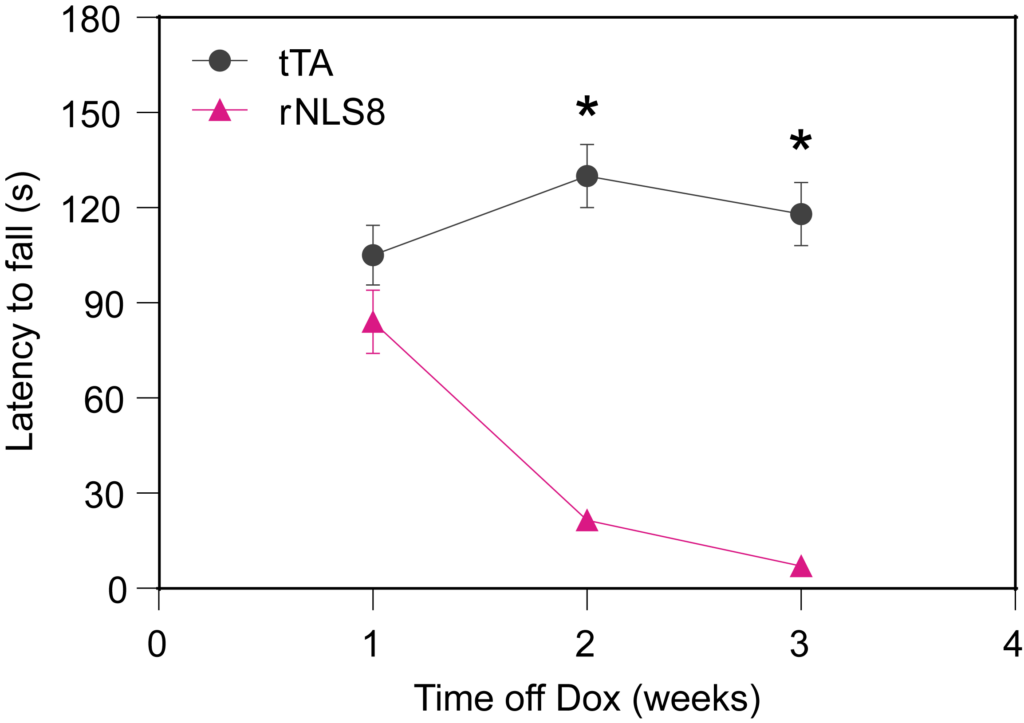
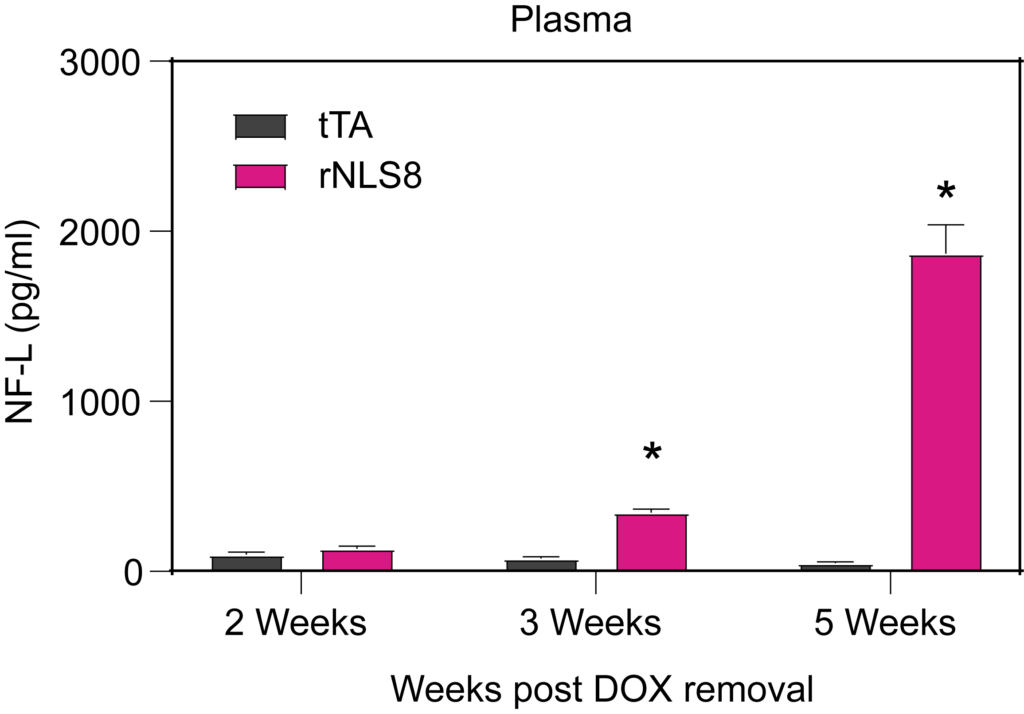
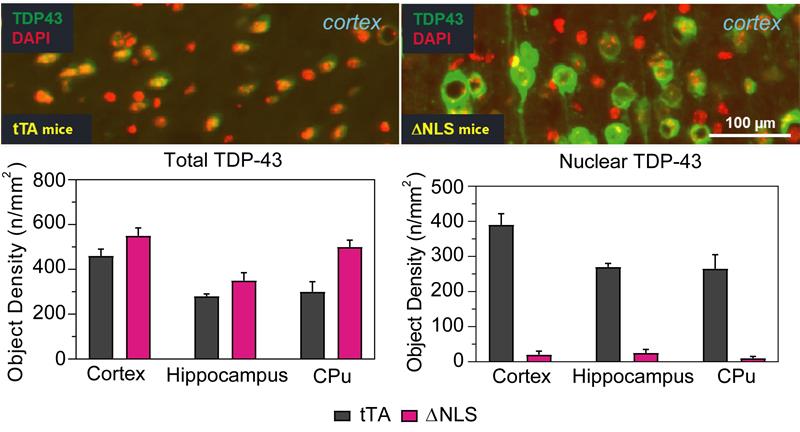
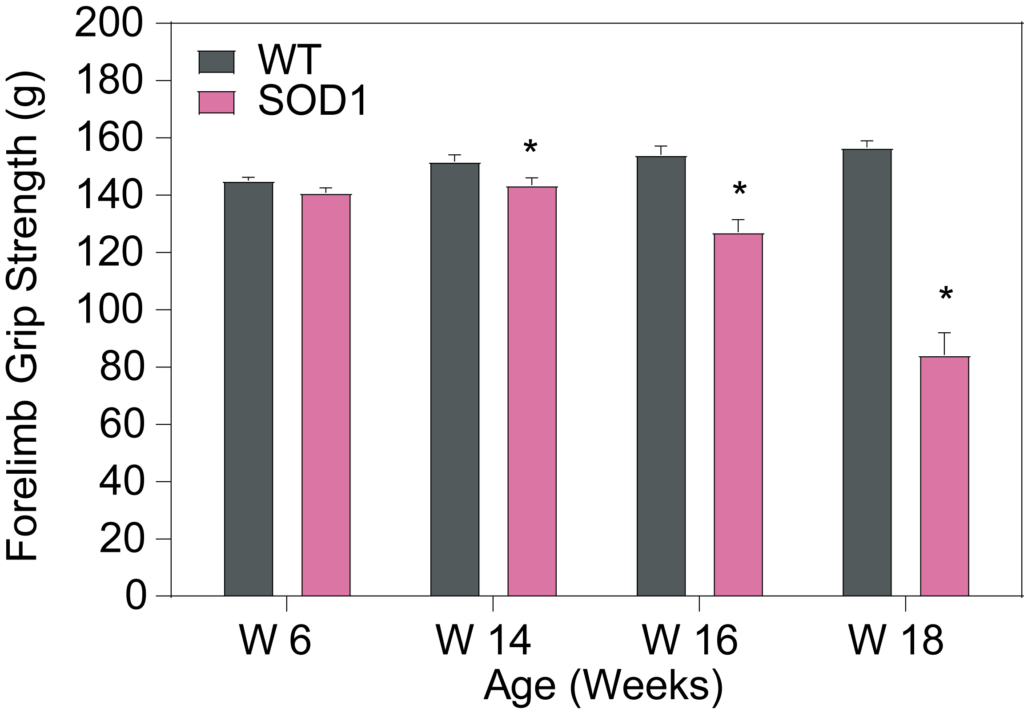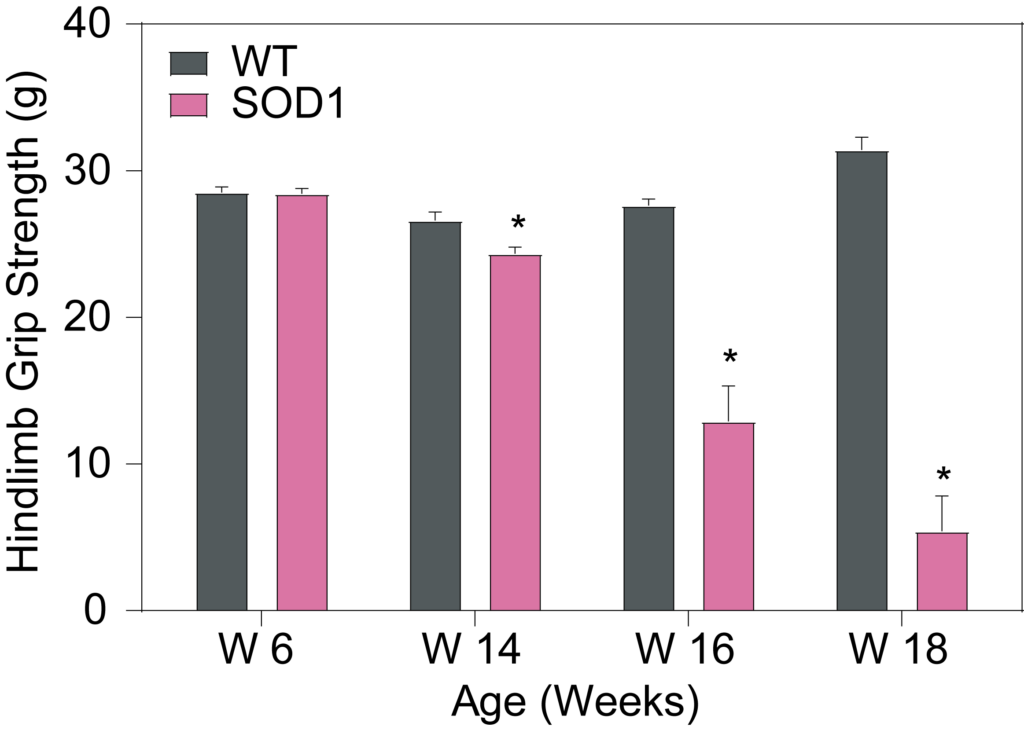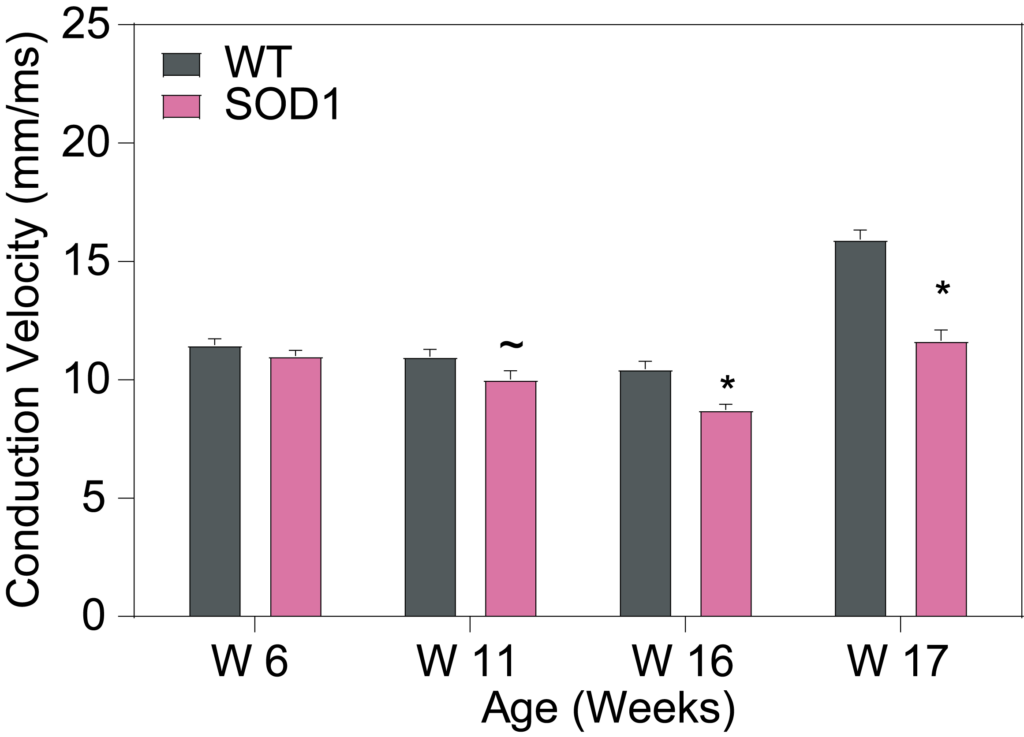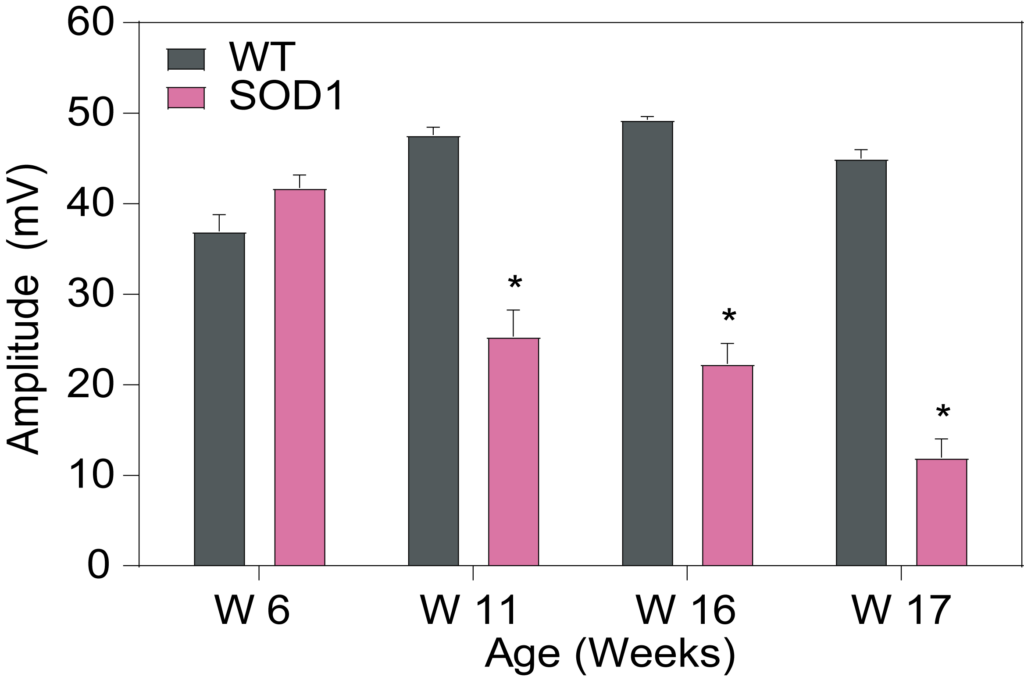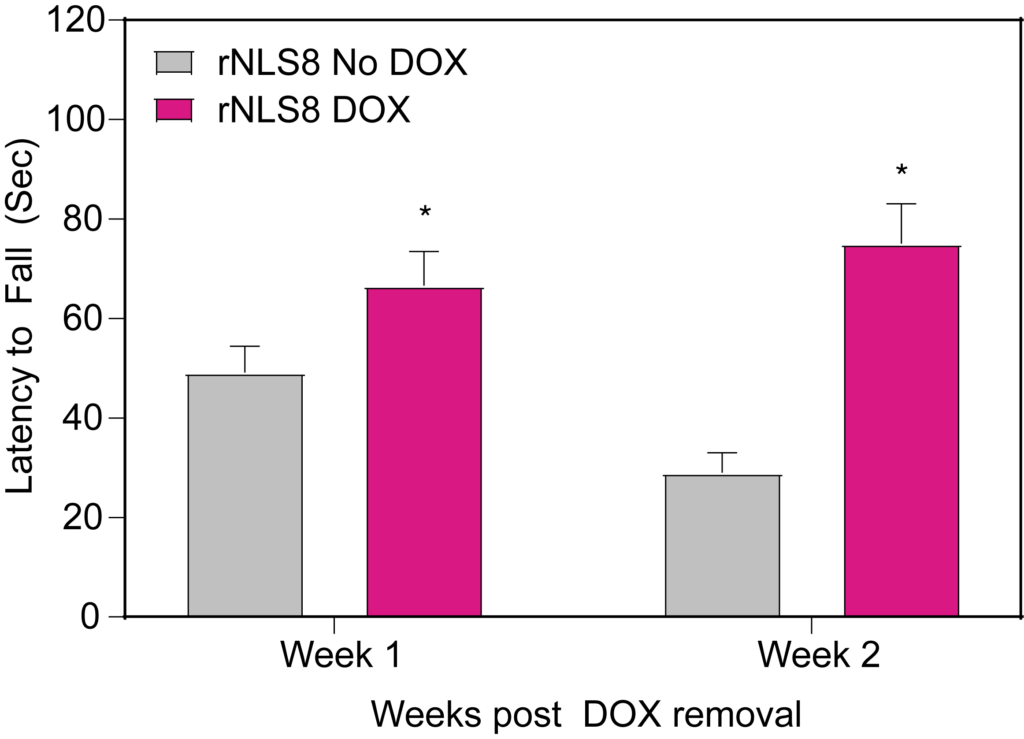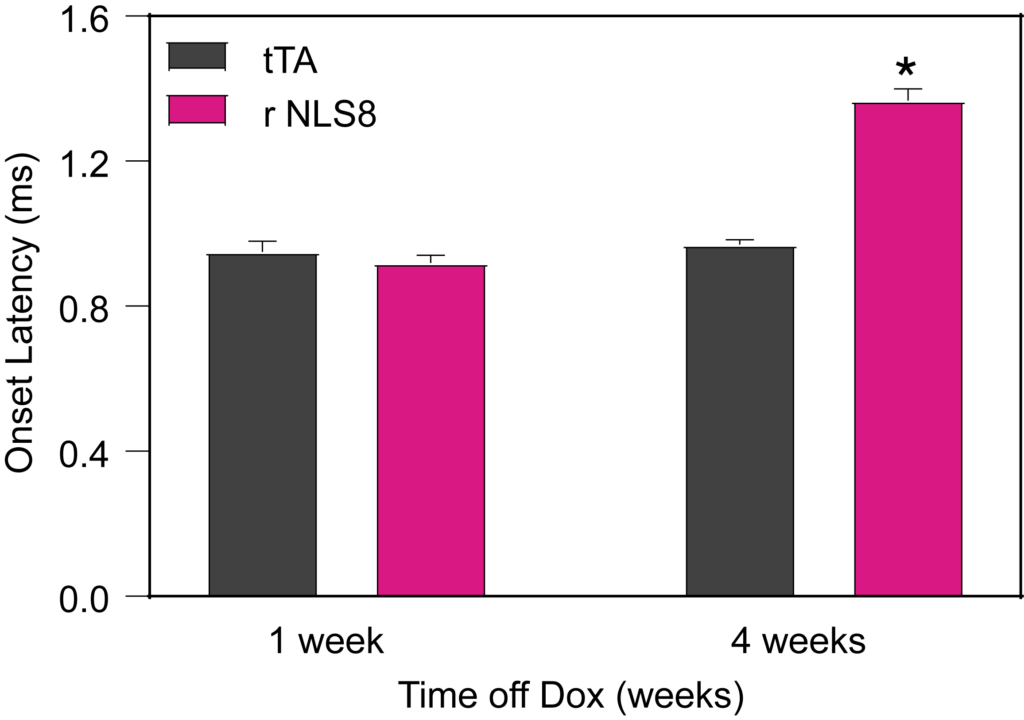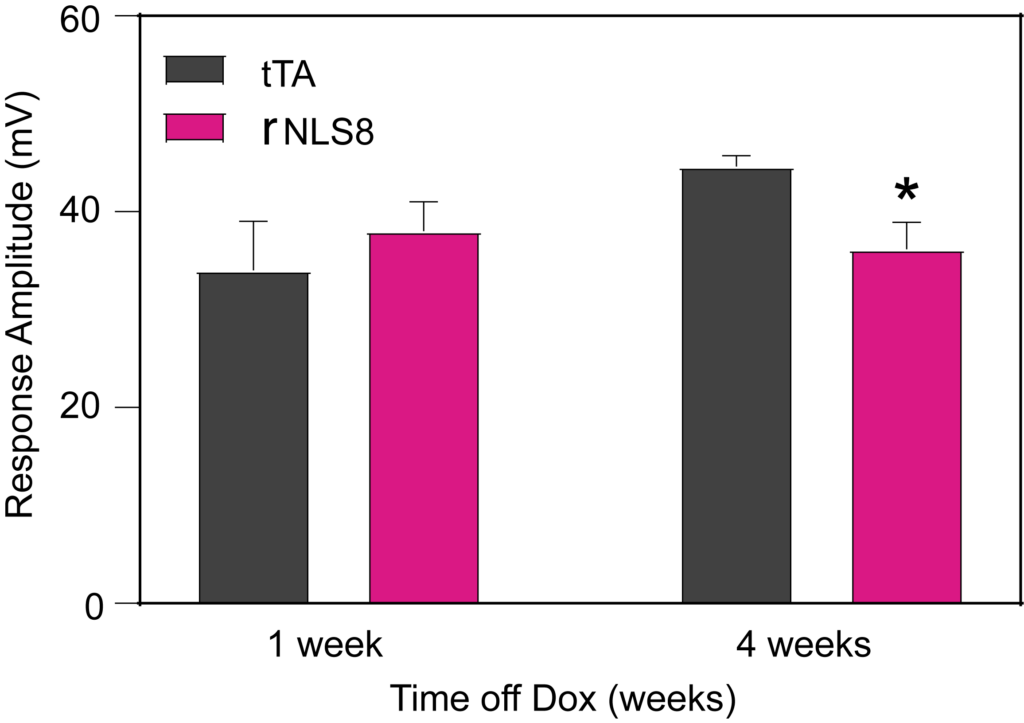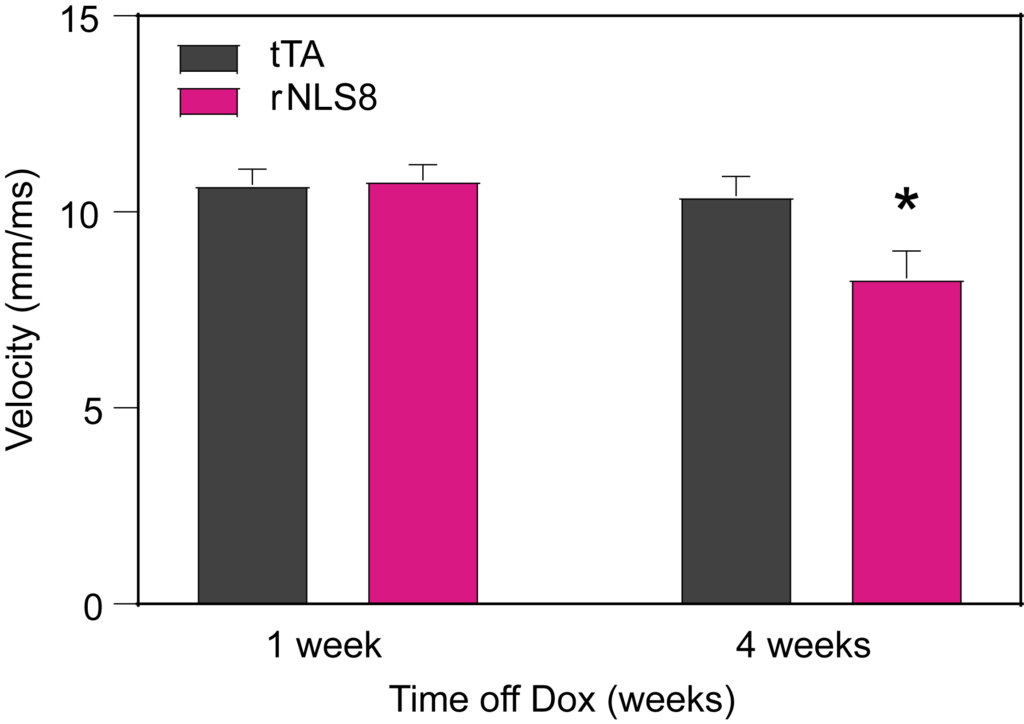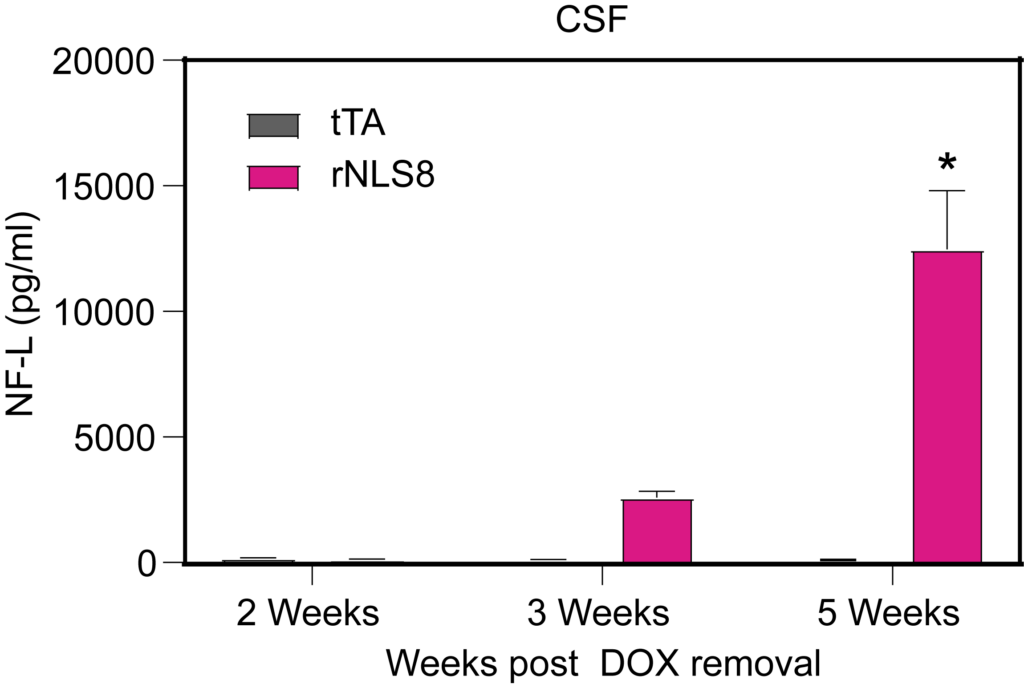PsychoGenics leverages transgenic models to advance preclinical studies of amyotrophic lateral sclerosis (ALS), a progressive paralytic disorder characterized by motor neuron degeneration in the brain and spinal cord. Our expertise extends to both sporadic (SALS) and familial (FALS) forms of ALS, with a particular focus on mutations in the superoxide dismutase 1 (SOD1) and TDP-43.
Partnering for Preclinical ALS Research Breakthroughs
PsychoGenics actively collaborates with researchers, pharmaceutical companies, and foundations committed to ALS research. Our extensive experience and specialized models provide a comprehensive and tailored approach to the preclinical development of your novel ALS therapy.
Collaborate with us to deepen our understanding of ALS pathogenesis and advance your therapeutic breakthrough, ultimately enhancing the lives of ALS patients worldwide.
Explore our areas of neurodegenerative disease specialization: